Introduction
The desire to understand and optimize facial beauty dates back as far as mankind, stemming in part from the innate biological drive to exude attractiveness and increase the likelihood of reproductive success. Certainly, beauty and facial expression play central roles in perception of emotional cues and other non-verbal communication. Societal and cultural factors, including media representations of ideal standards, in turn influence our perception of beauty and aesthetic goals. Many of these goals are aimed at combating signs of facial aging, including rhytides, volume depletion, and contour alterations, which have become associated with a decline in attractiveness.
In an effort to meet increasing and evolving consumer demand, numerous aesthetic tools have been developed to combat facial aging, restore normal anatomy, and optimize beauty. One such treatment is botulinum toxin type A (BTX-A), whom Justinus Kerner is credited with first identifying in 1820 (1,2). In 1980, the first report of therapeutic applications of BTX-A in humans demonstrated its ability to selectively weaken specific extraocular muscles and correct strabismus (3,4). It was not until 1992 that the first systematic study examining the cosmetic benefits of BTX-A was published, when Carruthers and Carruthers (5) showed its efficacy in treating glabellar rhytides. To date, five BTX-A products have been approved by the Food and Drug Administration (FDA): onabotulinumtoxinA (Botox, Allergan, Inc.), abobotulinumtoxinA (Dysport, Ipsen Biopharm Ltd.), incobotulinumtoxinA (Xeomin, Merz), prabotulinumtoxinA (Jeuveau, Evolus) and rimabotulinumtoxin B (Myobloc, Elan Pharmaceuticals) (6). All but the former are used for aesthetic purposes in the United States, and are FDA-approved for the treatment of moderate-to-severe glabellar lines at a minimum (7). Unless otherwise specified, units (U) mentioned in this chapter refer to onabotulinumtoxinA units.
The popularity of cosmetic procedures continues to rise, with Americans having spent 16.7 billion USD on cosmetic procedures in 2019, up from 13.5 billion USD in 2015 and a 237% increase compared to 2000 (8). Minimally-invasive procedures, including BTX-A and soft tissue augmentation, have continued to rise in popularity, representing 16.3 of the 18.1 million cosmetic procedures performed nationwide in 2019 (8). BTX-A represented nearly half of these minimally-invasive procedures (47.2%), highlighting its role as a mainstay of facial aesthetic treatments (8).
The upper third of the face, or specifically the region from the hairline to the upper eyelids, plays a key role in facial aesthetics and expression. In fact, BTX-A treatment has been associated with a more positive emotional state, increased level of satisfaction, better treatment by others, and improved overall quality of life, in addition to aesthetic benefits (6, 9-14). The formation of a genuine smile, also referred to as a Duchenne smile, requires activation of upper facial muscles (15). Duchenne smiles are differentiated from non-Duchenne smiles by orbicularis oculi muscle activation to reveal wrinkles around the eyes (also termed crow’s feet), in addition to sufficient activation of zygomaticus major to raise the cheeks, curve the mouth upwards, and show teeth (15). The glabella is the first area to be noticed in facial expression, depicting impatience, anger, and fatigue (16). The eyes and eyebrows are also frequently noticed facial features in daily interactions. Formation of dynamic rhytides over the forehead, glabella, and lateral canthal region can eventually lead to the development of static rhytides with repetitive muscle activity and dermal atrophy. These changes, along with eyebrow ptosis and under-projection, represent common features seen with aging. However, specific patient concerns are best interpreted in the context of the full facial anatomy, and not dealt with in isolation in order to achieve natural results. As we learn more about the complex dimensional aging process, with volume loss and bone resorption being prominent features, consideration should always be given to combination treatments.
Herein, we will explore the benefits of BTX-A in treating the upper third of the face through a discussion of upper facial beauty and aging, relevant anatomy, patient assessment, and injection technique. Through a holistic lens in the context of the entire face, we will additionally discuss how the use of BTX-A in the upper third of the face can help achieve a natural, aesthetically pleasing look.
Upper Facial Beauty and Aging
Although ideal features of specific facial components such as the eyebrows and eyelids may be considered in isolation, it is critical to note that the different facial regions are often interdependent and must move and function harmoniously. There can be considerable variability in what might be considered ideal size, shape, fullness, and position of individual facial features based on how they interplay with the surrounding features (17). Historically, mathematically idealized facial proportions, such as the concept of “Phi” or the golden ratio, have been considered synonymous with beauty (18). Since the time of ancient civilizations, “classical canons of beauty” were proposed in an attempt to define attractive facial features and ratios (19). More recently, it has been suggested that the classic vertical facial trisection cannon of upper face height as one-third, mid-face height as one-third and lower anterior face height as one third of total anterior face height, may be used as an ideal proportional ratio (20). However, it’s important to note that these proportions have largely been studied in Caucasians and that ideal aesthetics of the upper facial third are heavily influenced by factors such as age, gender, ethnicity, and societal perceptions of ideal standards (17).
For example, the ideal female eyebrow sits superior to the supraorbital rim, with a higher tail than in males and is arched with the apex at the lateral limbus, roughly two-thirds of the way along the brow (6, 21). The male brow is often at the level of the supraorbital rim with a more linear appearance (21). The ideal brow appearance has evolved over time to fit the perceptions of ideal standards, as evidenced by the descriptions of ideal brow peak by Westmore and Cook in the 1970s and 1980s, respectively (17). Age-related eyebrow underprojection and ptosis become increasingly evident secondary to volume loss, bone attenuation, and loss of cutaneous elasticity (6). This can be particularly apparent over the lateral brow due to a lack of naturally-occurring brow elevators laterally (6). Both eyebrow ptosis and upper eyelid volume loss can present as skin folds over the upper eyelids (6). Dermatochalasis is the non-specific term referring to any stretching or senile elastosis of upper eyelid skin that results in exaggerated eyelid folds (22). Aging changes of the upper eyelid also include a sulcus deformity of the upper medial eyelid, sometimes referred to as an ‘A-frame’ deformity or infrabrow hollow (6).
Our more nuanced understanding of facial aging has led to a greater appreciation for the multifactorial nature of this process. Specifically, more attention has been paid to the importance of volume loss and redistribution of subcutaneous fat. Cadaveric studies have demonstrated that facial subcutaneous fat is organized into distinct compartments that can redistribute, decrease in size, and age independently of one another (18, 23). For example, the forehead is composed of three anatomical units of subcutaneous fat; namely, the central, middle, and lateral temporal cheek fat (23). Redistribution and loss of fat can contribute to a sunken, flattened quality with a loss of the fullness and roundness of youth (18). Ultraviolet light-induced damage, pigmentary changes, alterations in facial musculature, bone and cartilage resorption, and loss of elasticity are also characteristic of the aging process. Rhytides are a key feature that contribute to an aged appearance, with a multifactorial etiology, including contributions from ultraviolet light-induced damage, repeated muscle movement over a lifetime, loss of skin elasticity, and volume loss secondary to fat redistribution and bone and cartilage resorption (18). Initially, rhytides may only appear with movement (dynamic rhytides); however, repetitive expressions secondary to muscle activity along with the other etiologic factors outlined above eventually result in rhytides present at rest (static rhytides). With aging, we also expect to see elongation of the upper third of the face due to recession of the frontal hairline and brow ptosis (18).
The ideal female forehead shows a gentle convexity of 12-15 degrees off the vertical as one goes from the supraorbital prominence to the hairline (6, 24). The male forehead tends to be both wider and taller, with a more prominent supraorbital ridge and a backward slope greater than that of females (17). With age, volume loss can lead to increased forehead concavity. Smooth contour transitions between the temple and forehead regions impart a youthful appearance, with convex temples forming a continuous contour with the forehead and zygomatic arch, without a sudden drop-off in volume at the temporal suture line (6). The desired degree of convexity varies with gender, with treatment aimed at producing more convex temples in males and a flat or mildly concave appearance in females (6). Similar to the forehead, advancing age can result in progressive temporal fossa concavity, leading to a more prominent bony outline of the lateral orbital rim(6). Aging changes in the glabellar region and lateral canthal zone may also include volume loss with rhytide formation. Addressing these changes is a common treatment goal.
Anatomy
Although a thorough discussion of facial anatomy is beyond the scope of this chapter, BTX-A treatment requires an appreciation of the underlying muscles of facial expression if one is to achieve aesthetically pleasing, natural outcomes. It is additionally critical to understand the trajectory of these muscles as they course from their origin to insertion, as this will impact the desired location and depth of injection, and ultimate cosmetic result. For the upper third of the face, the relevant muscles include frontalis, orbicularis oculi, procerus, corrugator supercilii, and depressor supercilii (Fig 1).
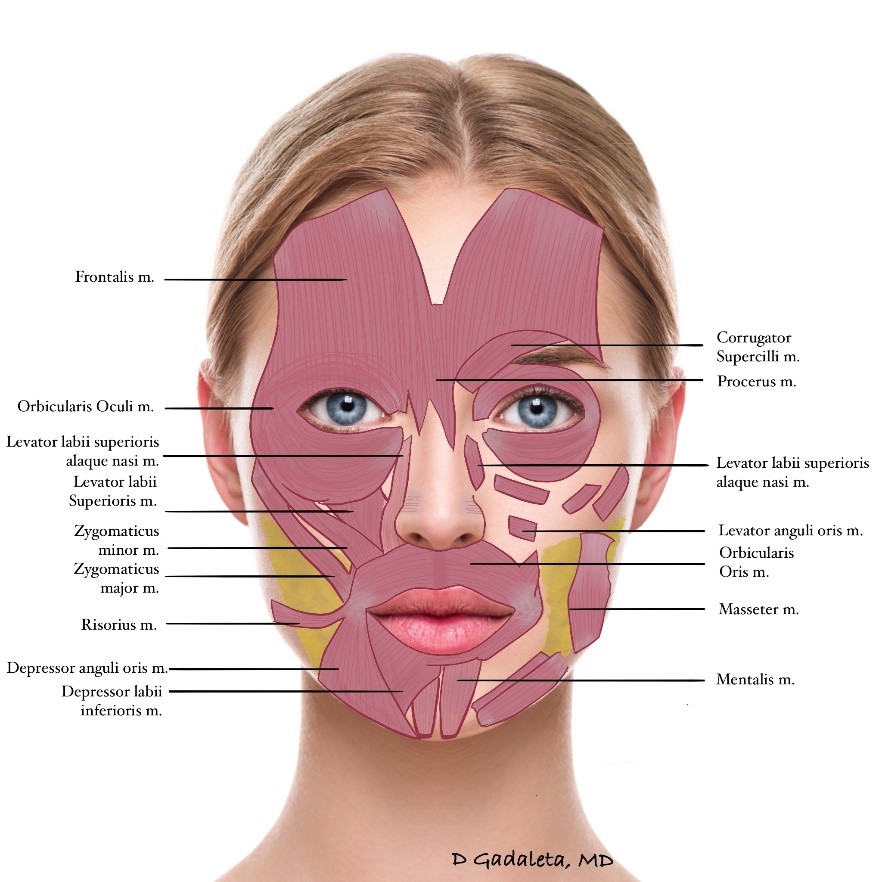
Frontalis is the predominant muscle underlying the forehead skin, and is the sole eyebrow elevator. The vertical orientation of its muscle fibers results in horizontal forehead lines with muscle contraction. Frequently quite symmetrical and rectangular in shape, the frontalis muscle originates cranially from the tendinous galea aponeurotica and travels at uniform depth beneath forehead skin (~3-5mm on average) to interdigitate with muscles between the eyebrows; namely, corrugator supercilii, procerus, depressor supercilii, and medial portions of orbicularis oculi (25). Laterally, the frontalis muscle terminates at the temporal fusion line. Of note, all these other muscles counteract the function of frontalis by collectively acting as eyebrow depressors (25).
Orbicularis oculi is a broad, flat oval-shaped muscle that encircles the eye and is comprised of orbital and palpebral parts. The orbital portion refers to the outermost portion of the muscle which overlies the orbital rim, originating from the nasal part of the frontal bone, frontal process of the maxilla, and the medial canthal tendon (22, 26). Superiorly, this portion interdigitates with the frontalis muscle, and acts as a sphincter muscle involved in voluntary eyelid closure (such as forced squeezing or winking) (22, 26). The palpebral portion of orbicularis lies within the upper and lower eyelids, and is further subdivided into the preseptal and pretarsal sections (27). The preseptal area lies in front of the orbital septum, originates from the medial palpebral ligament and inserts at the lateral palpebral raphe, and is involved in both voluntary eye closure and keeping the eyelids closed during sleep (26, 27). The innermost pretarsal portion arises from the medial canthal tendon and overlies the tarsal plates; this portion is primarily involved with involuntary blink closure (22, 26). The circular geometry of orbicularis oculi results in vertical muscle fiber orientation (resulting in horizontal wrinkling) at the lateral and medial points, and horizontal fiber orientation (vertical wrinkling) at the superior and inferior points, and angled fibers in between (25).
The procerus muscle is a thin, pyramidal-shaped depressor of the brow, which originates in fascia at the inferior nasal bone and becomes more superficial, inserting in the glabella where it interdigitates with the frontalis muscle (25). The procerus muscle depresses the medial portion of the brow, and also contributes to horizontal rhytides of the nasal dorsum (27).
Corrugator supercilii is another brow depressor that contributes to frowning, squinting, and vertical rhytides in the glabella and lower forehead (27). Its origin lies in the medial orbital rim and it runs superiorly and laterally to insert into the dermis, though the exact area of insertion is highly variable (25). Importantly, the corrugator supercilii muscle travels at different depths along its path, which must be considered in treatment planning (28). It is deepest at its bony origin and becomes progressively more superficial, eventually inserting into the dermis. Limiting BTX-A spread to surrounding muscles is particularly important in the glabellar region since the muscles here interdigitate and function together (28).
The depressor supercilii muscle pulls the medial eyebrow downwards, further contributing to frowning and squinting (25). Some consider this to be part of the medial orbicularis oculi muscle (29). It originates from the frontal process of the maxillary bone often as two distinct heads, typically 1cm above the medial canthal tendon (30). The insertion point is roughly 13-14mm superior to the medial canthal tendon, lying lateral to the insertion of corrugator supercilii (30).
Assessment
Initial Assessment
Creation of individualized treatment plans requires consideration of numerous factors, and distinguishing between what the patients wants and what professional judgement suggests is needed. The initial assessment should include consideration of static and dynamic features. Static considerations include eyebrow position and shape, forehead height, volume changes, overall facial symmetry, and presence of static rhytides. Palpation of muscle bulk and skin thickness is also important as these may impact the quantity and depth of BTX-A injection. Dynamic assessment is especially important because while facial anatomy is fairly preserved amongst individuals, the fashion in which muscles of facial expression are used may vary greatly (16, 31). Raising the eyebrows allows assessment of forehead wrinkles. Lateral canthal wrinkles are assessed by first smiling and then closing the eyes tightly without moving the cheeks. Both orbicularis and zygomaticus major muscles contribute to lateral canthal lines when making a full smile, while closing the eyes without smiling highlights rhytides arising from orbicularis oculi.
In general, neuromodulator treatment should be based on muscle bulk, distribution, and activity. Several of these components of the initial assessment will be touched on here, and all will be explored in greater depth during discussion of specific treatment sites for the upper third of the face.
Eyebrow position and shape
A key goal of upper face neuromodulator treatment is optimizing brow shape and position. Eyebrow position should be palpated in relation to the orbital rim. As mentioned, the eyebrows have one elevator (frontalis), which is counterbalanced by several brow depressors (corrugator supercilii, procerus, depressor supercilii and portions of orbicularis oculi) (19). Frontalis gives rise to horizontal forehead wrinkles, while corrugator and procerus cause vertical and horizontal glabellar rhytides, respectively (19). In order to create an individualized treatment plan, precise assessment of brow elevator and depressor activity is critical. For example, if the procerus muscle exerts significant downwards pull on the medial brow and creates a transverse lower glabellar rhytid, it would be important to inject that muscle if the goal of treatment is to achieve medial brow elevation. Conversely, if the frontalis muscle does not extend far laterally, then it may not be necessary to inject the forehead laterally.
Eyebrow ptosis may also manifest in the form of eyelid hooding or dermatochalasis. For example, if one fails to consider how much the frontalis muscle is contributing to holding up the brow when treating horizontal forehead rhytides, this may lead to inadvertent eyelid ptosis. This is particularly true in the setting of a dehisced levator palpebrae muscle and can be explained by Hering’s law, which states that both eyes behave as a single organ with equal and simultaneous innervation to the extraocular muscles (32). In the setting of eyelid ptosis, the ipsilateral levator palpebrae superioris muscle receives increased innervation that can result in compensatory contralateral eyelid retraction; often, the frontalis muscle compensates for eyelid ptosis, manifesting as unconscious ipsilateral brow elevation in patients with unilateral eyelid ptosis (32-34).It follows that treatment of frontalis in the setting of a dehisced levator palpebrae muscle is likely to cause or worsen eyelid ptosis. In patients with bilateral brow and eyelid ptosis, manual raising of the more severely affected brow results in inferior migration of the contralateral brow and worsening of eyelid hooding (34).
Symmetry
Assessment for overall symmetry in the upper third of the face is very important when considering forehead and glabellar injection patterns. Some degree of eyebrow asymmetry will commonly be noted, and, in such cases, a decision must be made as to whether to elevate the lower brow, lower the elevated one, or a combination of both.
Forehead height
Individuals with taller foreheads may require more than one row of BTX-A injections into the frontalis muscle to effectively treat horizontal forehead lines. More than one row of injections may also be considered when wrinkles extend into the hairline, such as in patients with a receded hairline. Conversely, those with a short forehead may require lower doses of BTX-A in order to avoid overly weakening the lower frontalis fibers, which can lead to eyebrow and eyelid ptosis.
Volume changes
Assessment of cutaneous thinning, fat atrophy and bone attenuation is an important aspect of the initial assessment when considering BTX-A treatment. Volume loss on the forehead, temples, and lateral canthal region can make it challenging to achieve optimal results with neuromodulators alone. As a result, this observation should prompt consideration of combination treatment, such as with soft tissue fillers.
Wrinkle pattern
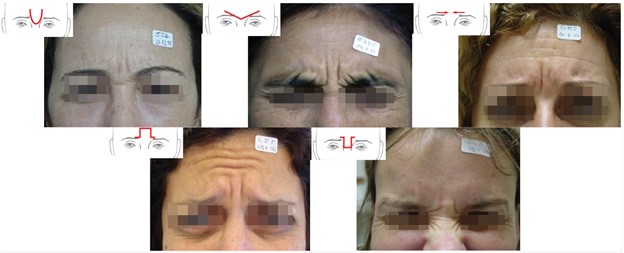
Common wrinkle-related concerns in the upper face include horizontal forehead lines, glabellar lines, and lateral canthal lines. Differences in individual anatomy and dynamic muscle movement can produce a variety of static and dynamic wrinkle patterns. These different patterns, in turn, require varying BTX-A injection patterns for effective treatment. The primary muscles involved in glabellar contraction include the corrugators, orbicularis oculi, procerus, and depressor supercilii, with occasional contributions from frontalis and nasalis (35). de Almeida and colleagues (16) introduced five types of glabellar contraction patterns; namely, the “U”, “V”, “converging arrows”, “omega”, and “inverted omega” patterns. Kim and colleagues (35) proposed an alternative classification in Korean patients, including the “U”, “11”, “X”, “Phi”, and “I” patterns.
Individual anatomy must be considered when treating horizontal forehead lines, including forehead height, degree of volume loss and frontalis muscle details. Variations in frontalis muscle fascicle angle can produce different degrees of wavy or straight horizontal forehead lines, suggesting that ‘one-size-fits-all’ treatment plans are best avoided (36). As noted above, because the frontalis muscle is the sole eyebrow elevator, treatment of this muscle may lead to eyebrow ptosis (36). In addition to considering forehead height (see above), it is important to note bimodal forehead movement as demonstrated by Cotofana and colleagues (36). They described lower forehead skin moving cranially with resultant eyebrow elevation, and upper forehead skin moving caudally, leading to hairline depression. The horizontal line of convergence (C-line), an invisible line, delineates the junction between cranial and caudal frontalis muscle movement. They postulated that injections above the C-line may mitigate the risk of iatrogenic brow ptosis (36). Jabbour and colleagues (37) noted that upper forehead injections prevented eyebrow ptosis but may be less effective for forehead line reduction. Understanding these concepts may permit more predictable outcomes when treating the forehead, and minimize the risk of forehead neuromodulator injections with regards to eyebrow ptosis.The frontalis muscle varies in both width and continuity across the forehead. Understanding frontalis muscle extent and activity is an important determinant in forehead injection pattern. For example, if the frontalis muscle extends quite far laterally, it’s important to consider treating that area, so long as doing so won’t excessively lower the brows. When some individuals raise their forehead and brows, they may have continuous horizontal wrinkles across the forehead without ‘waviness’. This corresponds to the presence of continuous frontalis muscle from end to end of the forehead. Other individuals have decussation of the frontalis muscle, such that a fibrous band connects the interrupted portions of the muscle centrally. In the latter individuals, forehead rhytides will appear ‘wavy’ or with a central ‘V’ type pattern. Thus, clinically, the presence of a ‘wavy’ forehead wrinkle pattern suggests some degree of frontalis muscle decussation; typically, the wider the V-pattern, the wider the decussation (38). When treating horizontal forehead rhytides in these individuals, neuromodulator should be placed directly above the peak of the brow to avoid excessive brow peaking with treatment.
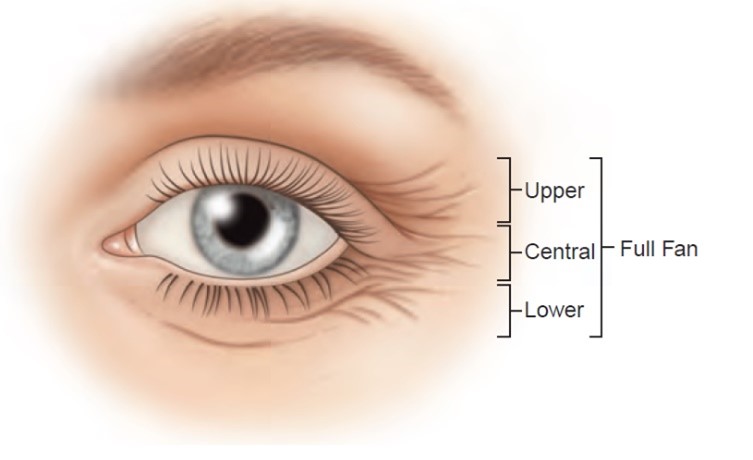
Kane (39) presented 4 patterns of lateral canthal rhytides; namely, a full-fan pattern (wrinkling from lower lateral brow across upper eyelid, through the lateral canthus), a lower-fan pattern (wrinkling in the lower lid and upper cheek area), a central-fan pattern (severe wrinkles only in the skin immediately surrounding the lateral canthus), and an upper-fan pattern (wrinkling only in the upper eyelid skin down to the lateral canthus) (40). The upper fan pattern is felt to be the least common both for static and dynamic lines at maximum smile, with some studies observing it in less than 5-10% of patients (40). Even within these 4 patterns, there is significant heterogeneity in lateral canthal rhytide patterns, highlighting the importance of tailoring treatment to the individual to optimize success and ensure patient satisfaction (40). The safety of BTX-A for treatment of lateral canthal rhytides has been well-established through multiple trials (41-43). There isn’t much data comparing neuromodulators for treatment of lateral canthal lines but one study found the safety and efficacy of prabotulinumtoxinA and onabotulinumtoxin to be comparable (44).
Pre-Treatment Evaluation and Patient Preparation
Product Selection and Reconstitution
While there is some head-to-head data comparing the various formulations of BTX-A, it is limited. In the authors’ experience, all approved products are effective and can be used to treat the various facial areas, albeit some in an off-label manner. The authors suggest becoming familiar with the performance of their chosen formulation and considering patient preference and history, as these are important factors in achieving excellent outcomes (45).
Given that dosing between the various BTX-A formulations is not interchangeable on a unit-to-unit basis, it is important to understand how each product performs. Unit conversions between the different formulations have been proposed, but this should not be considered definitive data (Table 1) (45-46). The previously held belief that some BTX-A formulations may be more effective than others has largely been debunked (46).
FDA labeling states that BTX-A formulations should be single-use, reconstituted only with sterile, preservative-free 0.9% sodium chloride, and stored in a refrigerator between 2° to 8°C upon reconstitution and administered within 24 hours of reconstitution (47). Unopened vials should also be stored in a refrigerator between 2° to 8°C with the exception of incobotulinumtoxinA which can be stored at room temperature up to 25°(48). These storage recommendations stem from concerns regarding potentially reduced potency and increased risk of infection, but sometimes present a financial burden to follow in a clinical setting (49).
Of note, Hexsel and colleagues (50) found that onabotulinumtoxinA may be used up to 6 weeks after reconstitution without loss of efficacy. Another study by Dressler and Bigalke (51) using a mouse hemidiaphragm assay showed that incobotulinumtoxinA showed no reduction of potency for up to 52 weeks after reconstitution. While this was not a human study, further study is warranted to confirm the prolonged potency beyond even 6 weeks. Furthermore, reconstitution with bacteriostatic, preservative-containing saline may improve treatment tolerability as benzyl alcohol acts as both a preservative and anesthetic (46, 49).
With regards to volume of reconstitution, most neuromodulator clinical trials use 2.5mL normal saline per 100 unit of neuromodulator except abobotulinumtoxinA trials which have used varying volumes per 300 or 500 unit vial. Having said that, the authors recommend differing volumes depending on the application. For example, smaller volumes of reconstitution may be preferable when precision is of utmost importance and diffusion to surrounding muscles is unwanted, such as in treatment of the glabella. Conversely, larger reconstitution volumes may be more appropriate when product spread is desired, such as in treatment of lateral canthal lines and large foreheads where eyebrow ptosis is not a concern. Carruthers and colleagues (50) found that treatment dilutions of 100, 33.3, 20, and 10 U/mL of onabotulinumtoxinA (20 female subjects per group) were all effective in improving glabellar rhytides; however, all 6 instances of eyebrow ptosis occurred in patients receiving more dilute injections (52). This suggests that caution should be exercised as larger volumes of reconstitution may lead to greater product spread, potentially inadvertently weakening unintended muscles, producing adverse outcomes.
Patient Preparation and Positioning
As with any treatment, patients should be screened for contraindications prior to BTX-A treatment. FDA-mandated contraindications pertinent to cosmetic treatment include hypersensitivity to any BTX-A preparation or to any components in the formulation, and infection at the proposed injection site (47). AbobotulinumtoxinA may contain trace amounts of cow’s milk protein; as a result, patients known to be allergic should avoid this formulation (53). Patients receiving concomitant treatment of BTX-A and aminoglycosides, or any other agents interfering with neuromuscular transmission (e.g. tetracyclines, polymyxins, penicillamine, anticholinesterases, and calcium channel blockers, amongst others), or muscle relaxants, are advised to do so with caution given possible potentiation of BTX-A effect (45, 47). Similarly, patients with neuromuscular disorders such as myasthenia gravis, Eaton-Lambert syndrome, and amyotrophic lateral sclerosis should ideally not be treated (53). BTX-A is pregnancy category C and the amount of excretion in breast milk of nursing mothers is unknown; as a result, it should be avoided in both pregnant and breastfeeding women (47). Individual physicians have differing levels of comfort in treating patients with disorders or medications affecting bleeding and clotting. Additional considerations include recognizing the presence of psychiatric disease and patients deemed to have unrealistic goals (53). Although zoster reactivation is felt to be a rare side effect of BTX-A treatment, it may prompt some practitioners to prescribe prophylactic antiviral medication, particularly for patients with a history of cold sores in the area to be treated (45).
Optimal patient positioning for treatment can be somewhat patient- and injector-dependent. Ideally, the patient will be seated upright with their head at the height of the injector’s shoulder and supported by a headrest (45). Alcohol pads can be used to remove makeup, facial oils, and debris from injection sites; chlorhexidine or other anti-septic products are more commonly reserved for dermal injections (45).
Analgesia and Needle Size
While minimizing patient discomfort is important in any procedure, it is of particular importance in an elective procedure such as BTX-A injection. BTX-A injections are generally well-tolerated with simple use of benzyl-alcohol-preserved normal saline and no other analgesia. However, some injectors prefer the addition of analgesic strategies such as use of ice packs, cooled needles, vibration anesthesia, and topical anesthetics (45, 54-55). Injection techniques to minimize pain can also include serial puncture technique with perpendicular alignment of needles (56) and pinching the skin firmly in the treatment area, making use of Melzack and Wall’s gate theory of pain.
While the FDA recommends use of 30- to 33-gauge needles for treatment of glabellar facial lines, 30-gauge needles are routinely used for BTX-A injections on the face (47, 53, 57-58). Flynn and colleagues (59) recommended use of the 0.3cc U-100 insulin syringe with 30-gauge short Ultra-Fine II needle (B-D Inc, Franklin Lakes, NJ) citing its ease of use, cost efficiency, small size, accuracy of toxin delivery, and minimization of pain. Although 32-gauge needles are thinner than 30-gauge needles, they are more expensive (58). Many have studied whether use of smaller-gauge needles might be worth the additional cost in exchange for reduced patient discomfort and bruising; however, results have been mixed with no consistent difference shown (54, 57-58). Of note, Price and colleagues (58) found that 6/37 subjects (16.2%) in a split-face study noted an increased BTX-A paralysis effect with use of a 32-gauge needle. Overall, no needle size has been found to be significantly superior in terms of treatment efficacy and patient comfort for BTX-A injection. The authors’ personal preference is the 0.3cc U-100 insulin syringe with 31-gauge Ultra-Fine II needle (B-D Inc, Franklin Lakes, NJ); in addition to the aforementioned benefits, this syringe lacks a hub, thus minimizing neuromodulator loss.
Treatment of the Upper Facial Third
Forehead
The most common indication for BTX-A treatment in the forehead is for smoothening of horizontal lines secondary to repeated frontalis contraction and forehead volume loss. Treatment of the forehead can have significant effects on brow shape and elevation (see below). Attempting to completely eliminate horizontal forehead lines may result in excess or inappropriate injections into frontalis, resulting in brow ptosis (see above) or an unnatural loss of expressiveness. To avoid the latter, treatment of frontalis should not completely eliminate the ability to elevate the eyebrows, although it will definitely be reduced. A global aesthetics consensus group recommends a total of 8 to 25 units (U) of onabotulinumtoxin A for treatment of horizontal forehead lines, via an intramuscular or intracutaneous approach (60). This can be done through 4 to 8 (non-microdroplet; 2 to 4 U onabotulinumtoxinA per injection point) or 8 to 20 injection points (microdroplet; 0.5 to 1.5 U onabotulinumtoxinA per injection point) (60). Some authors have devised complex treatment protocols to provide more specific injection guidance, taking into consideration variation in individual facial anatomy and clinical findings (61). Intracutaneous injections can be used as an additional tool to avoid eyebrow ptosis by achieving superficial distribution of toxin with lower dose per unit volume of frontalis muscle, particularly when injecting the lower forehead near the eyebrow(6).
General forehead treatment considerations include use of injection points higher up to decrease risk of eyebrow ptosis, and use of more than one row of injections for individuals with tall foreheads or dynamic rhytides extending to the frontal hairline (6). A randomized controlled trial compared the effect of three forehead injection patterns (V-pattern, middle horizontal pattern, and high horizontal pattern) and proposed an algorithmic approach (37). The authors suggested that patient’s whose primary concern is treatment of forehead lines while desiring to maintain brow depression should receive the V-injection pattern, as this was the most effective pattern for treating forehead lines while yielding less eyebrow ptosis than the middle horizontal pattern (37). For patients hoping to maintain good eyebrow height while treating the forehead lines they suggested the upper forehead injection pattern, recognizing that this pattern may not be as effective at reducing forehead wrinkle lines; two common scenarios for this pattern are young individuals with minimal wrinkles limited to the upper forehead, and older individuals with eyebrow ptosis compensated by baseline frontalis contraction (37). These findings are consistent with the line of convergence (see above), helping to achieve more predictable effects on eyebrow position. BTX-A injection into the medial frontalis may lead to a compensatory increase in the resting tone of the lateral frontalis fibers, resulting in elevation of the lateral eyebrow (37). The lateral extent of BTX-A injections on the forehead will depend largely on the width of the frontalis muscle, as noted in the dynamic assessment (6). Consideration should be given to combination treatment in the forehead, particularly when volume loss is present. This can present as mid-forehead or supra-brow concavity, particularly laterally, and is best treated with soft tissue fillers to restore the aesthetically appealing forehead convexity and to support the skin, reducing the formation of deeper rhytides, especially when the patient attempts to elevate the brows. Of note, BTX-A injections into frontalis and glabella have been found to effectively and safely produce a forehead lift; this effect was also noted to be greater in patients with short foreheads, for whom extending forehead height would be desirable (62).
Eyebrows
Common BTX-A treatment indications related to the eyebrows include adjustment of their position and/or shape. This may be due to age-related eyebrow ptosis, or a patient’s desire to optimize eyebrow appearance to better fit ideals based on age, gender, ethnicity, societal norms, and/or personal preferences. While brow ptosis is a concern with treatment of horizontal forehead lines, as previously noted BTX-A injection of the frontalis muscle can also be used to modify eyebrow position and shape therapeutically. Use of lower or higher injections on frontalis can be used to purposely lower or elevate the brow position, respectively. Use of a “V”- or “M”-shaped forehead injection pattern can enhance eyebrow arch, while a middle horizontal forehead pattern can be implemented to produce a straighter brow (6). If the lateral frontalis is insufficiently treated, the lateral eyebrows may remain raised and impart a peaked, diabolical or quizzical appearance.
Alternatively, patients desiring brow elevation without treatment of forehead lines may receive injection into the medial and/or lateral eyebrow depressors, without any frontalis injections (37). Every individual will likely have some degree of brow asymmetry; as a result, the number of injection points and amount of BTX-A delivered medially and laterally on each side of the face may not be equal.
For lateral brow elevation, one approach is to weaken the descending lateral orbicularis oculi muscle fibers. Asking the patient to close their eyes tightly can help identify the point of maximum contraction of the orbicularis oculi muscle that is causing lateral brow depression. The greatest benefit usually occurs if the brows are observed to move inferiorly with tight eye closure. In the author’s experience, superficial injection of 1U of BTX-A into 1-3 sites at the tail of each eyebrow can produce lateral brow elevation. Treatment of the upper lateral canthal lines (see below) with subdermal injection of 1 to 2 units of BTX-A can also enhance lateral brow elevation (6). Global aesthetics consensus group recommendations for lateral brow elevation include use of 1-2 intramuscular injection points bilaterally (0.5-1.5 U onabotulinumtoxinA per injection point) into the orbicularis oculi muscle at a point superior to the uppermost injection point for lateral canthal lines, typically at the eyebrow hairline (60). Medial brow elevation can be achieved by targeting the medial brow depressors; namely, corrugator supercilii, procerus, depressor supercilii, and medial portions of orbicularis oculi. This may include 1-2 bilateral intramuscular injections (0.5-4.0 U onabotulinumtoxinA per injection point) into procerus, corrugator supercilii, depressor supercilii, and orbicularis oculi (60). A randomized clinical trial by El-Khoury and colleagues (63) noted significant differences in eyebrow height at the level of the medial brow, medial canthus, and lateral brow edge when one group was injected in the medial and lateral eyebrow depressors and the second group was only injected in the latter. This further highlights the importance of injection pattern individualization based on patient needs and treatment goals.
Glabella
The glabellar complex is composed of several eyebrow depressor muscles; namely, the corrugator supercilii, procerus, depressor supercilii, as well as medial portions of the orbicularis oculi. As a result, neuromodulator treatment of glabellar rhytides should be approached in the context of the larger glabellar-forehead complex, and the factors noted above for brow position, shape, and the forehead should be simultaneously considered. In a clinical trial, 488 BTX-A-naïve patients observed significant improvement in moderate-to-severe glabellar frown lines with 10 or 20 units of Chinese botulinum toxin type A based on clinical assessment by blinded investigators; greater improvement was noted in the high-dose treatment group (64).
Consensus group recommendations for glabellar lines include intramuscular injections using 3 to 7 injection points (2 to 4 U onabotulinumtoxinA per injection point) targeting corrugator supercilii, procerus, depressor supercilii, as well as orbicularis oculi; typical total doses can vary from 12 to 40 U of onabotulinumtoxinA (60). The location of the injection points can vary based on the glabellar contraction pattern of the individual patient (see above), and should be at least 1cm above the orbital rim to prevent orbital diffusion and upper eyelid ptosis. However, care must also be taken to avoid frontalis muscle injection so as to reduce the likelihood of brow ptosis. This is best done by injecting superficially over the brows. First-time patients or less-experienced injectors should err on the side of conservative dosing in the glabella, to lessen the risk of eyelid and eyebrow ptosis, as well as eyebrow splaying (6). A greater dose and number of injection points will be indicated for patients with greater muscle bulk and muscle activity, respectively (6). When injecting the glabellar region, gripping procerus and the corrugator supercilii muscles between the nondominant thumb and index finger can help isolate the muscles and optimize accuracy of neuromodulator delivery (6).
Lateral Canthal Lines (Crow’s Feet)
BTX-A is a well-established, safe, and effective treatment for lateral canthal rhytides. Lateral canthal lines are caused by orbicularis oculi muscle activity and, to some extent, by zygomaticus muscle activity. Consensus recommendations for this indication include 1 to 5 intramuscular injection points per side targeting lateral orbicularis oculi muscle fibers (1 to 4 U onabotulinumtoxinA per injection point) (60). Typical total doses of onabotulinumtoxinA can range from 6 to 15 U per side, but doses as low as 4 U may be appropriate for some patients (60). Some panelists recommended avoiding a second row of injections for rhytides that extend toward the temporal hairline, while others advocated for using a second row in patients with severe sun damage or who have undergone cosmetic surgery such as face lifting (60). Injecting into the uppermost fibers of orbicularis oculi can also provide some brow elevation (60). Zygomaticus muscle activity also contributes to the inferior lateral canthal rhytides, and may be treated as well (6). However, when aiming to inject the inferolateral orbicularis oculi muscle, a lower dose per injection site should be considered to prevent unintended spread to the zygomaticus muscle, which could result in smile asymmetry and a shelf-like appearance when smiling (6). When treating the lateral canthal lines, some authors suggest pointing the needle away from the eyes with the needle bevel up; furthermore, the injection point should be at least 1cm lateral to the orbital rim to prevent diffusion of BTX-A into the orbit and avoid weakening of the extraocular muscles with resultant diplopia (6). Additionally, we suggest keeping injections superficial in the lateral canthal region as the orbicularis oculi muscle in this region is thin and superficial.
When investigating the use of BTX-A for treating the various patterns of crow’s feet (see above) two injection patterns were available to investigators in the two phase 3 clinical trials (42). For the central-, upper-, and full-fan patterns, the first injection is in the orbicularis oculi muscle at the level of the lateral canthus, with the second and third injections located 1.0-1.5cm above and below the first injection point, respectively, at a 30° angle medially (42). For the lower-fan pattern, the first injection is in the same location, while the second and third injection points inferior to this form a line angling from superoposterior to anterioinferior (42). As previously mentioned, treatment should be customized based on the individual rhytide pattern and muscle bulk.
Temple Hypertrophy
While age-related temporal volume loss and concavity is an issue that is increasingly addressed with soft tissue fillers, in some individuals the converse issue arises. Ttemporalis muscle hypertrophy can lead to prominence and bulging of the temples and a disproportionately large appearance of the upper face. While less common, BTX-A has been used to reduce temporalis muscle hypertrophy. Jung and colleagues noted a decrease in temporalis muscle thickness from 6.2(+/-2.2) mm prior to treatment to 2.8(+/-1.7) mm one month after treatment following BTX-A (25 units/0.5mL) administration at five injection points (53). Upper face circumference decreased from 50.2(+/-9.2) cm to 49.1(+/-10.7) cm across the same time frame (65).
Hypertrophic Orbicularis Oculi
A hypertrophic orbicularis oculi muscle can manifest clinically as a prominent infraorbital muscular ridge, with lower eyelid rhytides or folds. This can be evident at rest and become more prominent with smiling, when contraction of the pretarsal orbicularis oculi muscle decreases the size of the palpebral aperture and gives a “jelly roll” appearance to the lower eyelid; affected patients may complain of appearing overweight (66). Many patients may also find eye widening attractive as it gives the lower eyelid a more rounded appearance (67). Flynn and colleagues (68) treated 15 female patients with BTX-A into the orbicularis oculi muscle. Both lower eyelids were treated with 2U BTX-A, while a single lateral canthal region was treated with 12U with 3 injections of 4U spaced 1cm apart. A synergistic effect was noted with concomitant treatment of the lateral canthal lines. Later, Flynn and colleagues (67) reproduced these results using different doses of BTX-A. 11 patients and 8 patients had 4U and 8U injected into the lower eyelid bilaterally, respectively. Once again, all patients also received 12U injected into a unilateral lateral canthal region. A dose-response effect with BTX-A for improvement of lower eyelid rhytides was noted. Simultaneous treatment of the lateral canthal lines had a synergistic effect at lower doses that plateaued at the higher dose. Of note, a hypertrophic orbicularis oculi muscle is a normal feature seen in many individuals, and complete elimination may be aesthetically undesirable.
After-Procedure Care
Standardized evidence-based recommendations on optimal post-procedure care are somewhat lacking. Some have recommended that patients exercise injected muscles for up to 4 hours after treatment to enhance cellular uptake (69-70). This is based on the premise that muscular activity may accelerate the binding of toxin to the cholinergic receptor (69, 71). With this in mind, it is possible that facial exercise may only be indicated for 1 hour as most binding of toxin will be complete by that time (69). Data supporting this recommendation is limited to a single small trial in which an earlier onset of clinical effect was observed when facial exercises were performed post-injection of BTX-A (70). Significant improvement in dynamic glabellar and static forehead wrinkles was noted 1 day earlier when injections were followed by facial exercises; however, the overall degree of effect was the same in both exercise and control groups at 14 days. The exercise regimen in this study consisted of 3 sets, each separated by 10 minutes, of 40 forehead raises followed by 40 scowls, or knitting of the brows, while the control group was asked to refrain from facial contractions for 4 hours. Overall, facial exercise after treatment with BTX-A is a safe intervention that may possibly lead to an earlier onset in clinical improvement, and may be recommended to patients for whom this is important (70). Given that radiolabelling studies have shown that BTX-A binds to nerves of actively contracting muscles within an average of 32 to 64 minutes, asking patients to perform facial exercises and remain upright for 1 hour may be more logical than the frequently recommended 4 hours (69, 71). Patients are also commonly asked to avoid massaging the treated area after injection to prevent unwanted product spread beyond the treated area (72). This recommendation is similarly felt to be low risk but there is no data to support this practice.
Conclusion
Neuromodulators are an excellent treatment option for the upper face. Understanding functional anatomy of the underlying muscles of facial expression is key if one is to achieve aesthetically pleasing, natural outcomes. Similarly, while clinical trials rely on standardized treatment protocols, optimal outcomes may be best achieved when treatments are tailored to the individual patient, based on anatomy, assessment, and treatment goals.
References
- Erbguth FJ, Naumann M. Historical aspects of botulinum toxin: Justinus Kerner (1786-1862) and the “sausage poison.” Neurology. 1999;53:1850–1853.
- Erbguth FJ. From poison to remedy: the chequered history of botulinum toxin. J Neural Transm (Vienna). 2008;115:559–565.
- Carruthers A, Carruthers J. Botulinum toxin type A: history and current cosmetic use in the upper face. InSeminars in cutaneous medicine and surgery 2001 Jun (Vol. 20, No. 2, pp. 71-84).
- Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Journal of pediatric ophthalmology and strabismus. 1980 Jan 1;17(1):21-5.
- Carruthers JD, Carruthers JA. Treatment of glabellar frown lines with C. botulinum‐A exotoxin. The Journal of dermatologic surgery and oncology. 1992 Jan;18(1):17-21.
- Bertucci V, Almohideb M, Pon K. Approaches to Facial Wrinkles and Contouring. In: Kantor J, ed. Dermatologic Surgery. New York: McGraw-Hill Education; 2018. P. 1243-70.
- United States Food and Drug Administration. https://www.fda.gov/media/89195/download. Accessed August 9, 2020.
- American Society for Aesthetic Plastic Surgery. 2019 ASAPS Statistics: Complete charts. https://www.plasticsurgery.org/documents/News/Statistics/2019/plastic-surgery-statistics-full-report-2019.pdf. Accessed August 2, 2020.
- Fagien S, Carruthers JD. A comprehensive review of patient-reported satisfaction with botulinum toxin type a for aesthetic procedures. Plastic and reconstructive surgery. 2008 Dec 1;122(6):1915-25.
- Alam, Murad, et al. “Botulinum toxin and the facial feedback hypothesis: Can looking better make you feel happier?.” (2008): 1061-1072.
- Heckmann M, Teichmann B, Schröder U, Sprengelmeyer R, Ceballos-Baumann AO. Pharmacologic denervation of frown muscles enhances baseline expression of happiness and decreases baseline expression of anger, sadness, and fear. Journal of the American Academy of Dermatology. 2003 Aug 1;49(2):213-6.
- Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatologic Surgery. 2006 May;32(5):645-50.
- Sommer B, Zschocke I, Bergfeld D, Sattler G, Augustin M. Satisfaction of patients after treatment with botulinum toxin for dynamic facial lines. Dermatologic surgery. 2003 May;29(5):456-60.
- Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. Journal of cosmetic dermatology. 2009 Mar;8(1):24-6.
- Soussignan R. Duchenne smile, emotional experience, and autonomic reactivity: a test of the facial feedback hypothesis. Emotion. 2002 Mar;2(1):52.
- de Almeida AR, da Costa Marques ER, Banegas R, Kadunc BV. Glabellar contraction patterns: a tool to optimize botulinum toxin treatment. Dermatologic surgery. 2012 Sep;38(9):1506-15.
- Sedgh J. The aesthetics of the upper face and brow: male and female differences. Facial Plastic Surgery. 2018 Apr;34(02):114-8.
- Wesley, N.O., & Rohrer, T. E. (2018). Evaluation of Beauty and the Aging Face. In Bolognia, J., Schaffer, J.V., & Cerroni, L (Ed.). Dermatology (4th Ed., 2571-2577). Philadelphia: Elsevier.
- Naini FB. Facial proportions: classical canons to modern craniofacial anthropometry. In: Naini FB (ed.). Facial aesthetics: concepts and clinical diagnosis. Oxford: Wiley-Blackwell, 2011, 18–44.
- Naini, F. B., Donaldson, A. N. A., McDonald, F., & Cobourne, M. T. (2013). How does variation in lower anterior face height influence perceived attractiveness? A quantitative investigation. Journal of Orthodontics, 40(3), 206-217.
- Lighthall JG. Rejuvenation of the upper face and brow: neuromodulators and fillers. Facial Plastic Surgery. 2018 Apr;34(02):119-27.
- Patrinely JR, Anderson RL. Anatomy of the orbicularis oculi and other facial muscles. Advances in neurology. 1988;49:15.
- Rohrich RJ, Pessa JE. The Fat Compartments of the Face: Anatomy and Clinical Implications for Cosmetic Surgery: Reply. Plastic and Reconstructive Surgery. 2008 Mar 1;121(3):1061-2.
- Muhn C, Rosen N, Solish N, Bertucci V, Lupin M, Dansereau A, Weksberg F, Remington BK, Swift A. The evolving role of hyaluronic acid fillers for facial volume restoration and contouring: a Canadian overview. Clinical, Cosmetic and Investigational Dermatology. 2012;5:147.
- Lorenc ZP, Smith S, Nestor M, Nelson D, Moradi A. Understanding the functional anatomy of the frontalis and glabellar complex for optimal aesthetic botulinum toxin type A therapy. Aesthetic plastic surgery. 2013 Oct 1;37(5):975-83.
- Marur T, Tuna Y, Demirci S. Facial anatomy. Clinics in dermatology. 2014 Jan 1;32(1):14-23.
- Bentsianov B, Blitzer A. Facial anatomy. Clinics in dermatology. 2004 Jan 1;22(1):3-13.
- Lee HJ, Lee KW, Tansatit T, Kim HJ. Three‐Dimensional Territory and Depth of the Corrugator Supercilii: Application to Botulinum Neurotoxin Injection. Clinical Anatomy. 2020 Jul;33(5):795-803.
- Wieder, J. M., & Moy, R. L. (1998). Understanding botulinum toxin: surgical anatomy of the frown, forehead, and periocular region. Dermatologic surgery, 24(11), 1172-1174.
- Cook Jr BE, Lucarelli MJ, Lemke BN. The depressor supercilii muscle: anatomy, histology, and cosmetic implications. The American Journal of Cosmetic Surgery. 2000 Dec;17(4):193-205.
- Fagien S, Raspaldo H. Facial rejuvenation with botulinumneurotoxin: an anatomical and experiential perspective. J Cosmetand Laser Ther 2007;9(Suppl 1):23–31.
- Chen AD, Lai YW, Lai HT, Huang SH, Lee SS, Chang KP, Lai CS. The impact of Hering’s law in blepharoptosis: literature review. Annals of plastic surgery. 2016 Mar 1;76:S96-100.
- Mehta HK. The contralateral upper eyelid in ptosis: some observations pertinent to ptosis corrective surgery. British Journal of Ophthalmology. 1979 Feb 1;63(2):120-4.
- Teske SA, Kersten RC, Devoto MH, Kulwin DR. Hering’s law and eyebrow position. Ophthalmic plastic and reconstructive surgery. 1998 Mar 1;14(2):105-6.
- Kim HS, Kim C, Cho H, Hwang JY, Kim YS. A study on glabellar wrinkle patterns in Koreans. Journal of the European Academy of Dermatology and Venereology. 2014 Oct;28(10):1332-9.
- Cotofana S, Freytag DL, Frank K, Sattler S, Landau M, Pavicic T, Fabi S, Lachman N, Hernandez CA, Green JB. The Bidirectional Movement of the Frontalis Muscle: Introducing the Line of Convergence and Its Potential Clinical Relevance. Plastic and reconstructive surgery. 2020 May 1;145(5):1155-62.
- Jabbour SF, Awaida CJ, ElKhoury JS, Rayess YA, Makhoul RB, Kechichian EG, Nasr MW. The impact of upper face botulinum toxin injections on eyebrow height and forehead lines: A randomized controlled trial and an algorithmic approach to forehead injection. Plastic and Reconstructive Surgery. 2018 Nov 1;142(5):1212-7.
- Moqadam, M., Frank, K., Handayan, C., Hakami, M., Benslimane, F., Gotkin, R. H., … & Cotofana, S. (2017). Understanding the Shape of Forehead Lines. Journal of Drugs in Dermatology: JDD, 16(5), 471-477.
- Kane MA. Classification of crow’s feet patterns among Caucasian women: the key to individualizing treatment. Plastic and reconstructive surgery. 2003 Oct 1;112(5):33S-9S.
- Kane MA, Cox SE, Jones D, Lei X, Gallagher CJ. Heterogeneity of crow’s feet line patterns in clinical trial subjects. Dermatologic Surgery. 2015 Apr 1;41(4):447-56.
- Dayan S, Coleman III WP, Dover JS, De Boulle K, Street J, Romagnano L, Daniels S, Kowalski JW, Lei X, Lee E. Effects of OnabotulinumtoxinA treatment for crow’s feet lines on patient-reported outcomes. Dermatologic Surgery. 2015 Jan 1;41:S67-74.
- Carruthers A, Bruce S, Cox SE, Kane MA, Lee E, Gallagher CJ. OnabotulinumtoxinA for treatment of moderate to severe crow’s feet lines: a review. Aesthetic Surgery Journal. 2016 May 1;36(5):591-7.
- Moers-Carpi M, Carruthers J, Fagien S, Lupo M, Delmar H, Jones D, Somogyi C, Lee E, Lei X, MacKinnon S, Davis PG. Efficacy and safety of onabotulinumtoxinA for treating crow’s feet lines alone or in combination with glabellar lines: a multicenter, randomized, controlled trial. Dermatologic Surgery. 2015 Jan 1;41(1):102-12.
- Cheon HI, Jung N, Won CH, Kim BJ, Lee YW. Efficacy and Safety of Prabotulinumtoxin A and Onabotulinumtoxin A for Crow’s Feet: A Phase 3, Multicenter, Randomized, Double-Blind, Split-Face Study. Dermatologic Surgery. 2019 Dec 1;45(12):1610-9.
- Erickson BP, Lee WW, Cohen J, Grunebaum LD. The role of neurotoxins in the periorbital and midfacial areas. Facial Plastic Surgery Clinics. 2015 May 1;23(2):243-55.
- Dover JS, Monheit G, Greener M, Pickett A. Botulinum toxin in aesthetic medicine: myths and realities. Dermatologic Surgery. 2018 Feb;44(2):249.
- United States Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/103000s5232lbl.pdf. Accessed August 17, 2020.
- United States Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125360s073lbl.pdf. Accessed August 17, 2020.
- Liu A, Carruthers A, Cohen JL, Coleman III WP, Dover JS, Hanke CW, Moy RL, Ozog DM. Recommendations and current practices for the reconstitution and storage of botulinum toxin type A. Journal of the American Academy of Dermatology. 2012 Sep 1;67(3):373-8.
- Hexsel, D. M., De Almeida, A. T., Rutowitsch, M., De Castro, I. A., Silveira, V. L. B., Gobatto, D. O., … & Zechmeister, D. (2003). Multicenter, double‐blind study of the efficacy of injections with botulinum toxin type A reconstituted up to six consecutive weeks before application. Dermatologic surgery, 29(5), 523-529.
- Dressler, D., & Bigalke, H. (2017). Long-term stability of reconstituted incobotulinumtoxinA: how can we reduce costs of botulinum toxin therapy?. Journal of Neural Transmission, 124(10), 1223-1225.
- Carruthers A, Carruthers J, Cohen J. Dilution volume of botulinum toxin type A for the treatment of glabellar rhytides: does it matter?. Dermatologic surgery. 2007 Jan;33:S97-104.
- Tremaine AM, McCullough JL. Botulinum toxin type A for the management of glabellar rhytids. Clinical, cosmetic and investigational dermatology: CCID. 2010;3:15.
- Yomtoob DE, Dewan MA, Lee MS, Harrison AR. Comparison of pain scores with 30-gauge and 32-gauge needles for periocular botulinum toxin type a injections. Ophthalmic Plastic & Reconstructive Surgery. 2009 Sep 1;25(5):376-7.
- Sharma P, Czyz CN, Wulc AE. Investigating the efficacy of vibration anesthesia to reduce pain from cosmetic botulinum toxin injections. Aesthetic surgery journal. 2011 Nov 1;31(8):966-71.
- Alam M, Tung R. Injection technique in neurotoxins and fillers: indications, products, and outcomes. Journal of the American Academy of Dermatology. 2018 Sep 1;79(3):423-35.
- Alam M, Geisler A, Sadhwani D, Goyal A, Poon E, Nodzenski M, Schaeffer MR, Tung R, Minkis K. Effect of needle size on pain perception in patients treated with botulinum toxin type A injections: a randomized clinical trial. JAMA dermatology. 2015 Nov 1;151(11):1194-9.
- Price KM, Williams ZY, Woodward JA. Needle preference in patients receiving cosmetic botulinum toxin type A. Dermatologic surgery. 2010 Jan;36(1):109-12.
- Flynn TC, Carruthers A, Carruthers J. Surgical pearl: the use of the Ultra-Fine II short needle 0.3-cc insulin syringe for botulinum toxin injections. Journal of the American academy of dermatology. 2002 Jun 1;46(6):931-3.
- Sundaram H, Signorini M, Liew S, de Almeida AR, Wu Y, Braz AV, Fagien S, Goodman GJ, Monheit G, Raspaldo H, Group GA. Global aesthetics consensus: botulinum toxin type A—evidence-based review, emerging concepts, and consensus recommendations for aesthetic use, including updates on complications. Plastic and reconstructive surgery. 2016 Mar;137(3):518.
- de Sanctis Pecora, C. (2020). One21: A Novel, Customizable Injection Protocol for Treatment of the Forehead with IncobotulinumtoxinA. Clinical, Cosmetic and Investigational Dermatology, 13, 127.
- Cohen S, Artzi O, Heller L. Forehead lift using botulinum toxin. Aesthetic surgery journal. 2018 Feb 15;38(3):312-20.
- El-Khoury JS, Jabbour SF, Awaida CJ, Rayess YA, Kechichian EG, Nasr MW. The impact of botulinum toxin on brow height and morphology: A randomized controlled trial. Plastic and Reconstructive Surgery. 2018 Jan 1;141(1):75-8.
- Feng Z, Sun Q, He L, Wu Y, Xie H, Zhao G, Xu J, Yao C, Li H. Optimal dosage of botulinum toxin type A for treatment of glabellar frown lines: efficacy and safety in a clinical trial. Dermatologic Surgery. 2015 Jan 1;41:S56-63.
- Jung GS. Temporalis muscle reduction using botulinum toxin type A for a desirable upper face circumference. Facial Plastic Surgery. 2019 Oct;35(05):559-60.
- Carruthers, A., Carruthers, J., & De Almeida, A. (2018). Botulinum Toxin. In J. Bolognia, J. V. Schaffer, & L. Cerroni (Authors), Dermatology (pp. 2661-2674). Philadelphia: Elsevier.
- Flynn, T. C., Carruthers, J. A., Carruthers, J. A., & Clark, R. E. (2003). Botulinum A toxin (BOTOX) in the lower eyelid: dose‐finding study. Dermatologic Surgery, 29(9), 943-951.
- Flynn, T. C., Carruthers, J. A., & Carruthers, J. A. (2001). Botulinum‐A toxin treatment of the lower eyelid improves infraorbital rhytides and widens the eye. Dermatologic surgery, 27(8), 703-708.
- Hsu TS, Dover JS, Kaminer MS, Arndt KA, Tan MH. Why make patients exercise facial muscles for 4 hours after botulinum toxin treatment?. Archives of dermatology. 2003 Jul 1;139(7):948-.
- Alam M, Geisler A, Warycha M, Paghdal K, Roongpisuthipong W, Schlessinger DI, Chen BR, Reynolds KA, West DP, Poon E. Effect of postinjection facial exercise on time of onset of botulinum toxin for glabella and forehead wrinkles: A randomized, controlled, crossover clinical trial. Journal of the American Academy of Dermatology. 2019 Apr 1;80(4):1144-7.
- Huang W, Foster JA, Rogachefsky AS. Pharmacology of botulinum toxin. Journal of the American academy of dermatology. 2000 Aug 1;43(2):249-59.
- Kordestani R, Small KH, Rohrich RJ. Advancements and refinement in facial neuromodulators. Plastic and Reconstructive Surgery. 2016 Oct 1;138(4):803-6.
