Introduction
The placement of injectable fillers has become one of the most popular nonsurgical, cosmetic procedures for facial rejuvenation over last decade. While considered to be a relatively safe procedure, there are definite risks associated with dermal filler injections, ranging from mild to severe. Though these complications are rare and generally minor, there are more serious risks that clinicians must be able to recognize and treat immediately. The most dramatic of these complications results from inadvertent intra-arterial filler injection, leading to vascular occlusion and subsequent tissue necrosis and blindness.1 While this is thought to be a rare occurrence, the true incidence is unknown due to clinician underreporting.2 Given that most patients request such procedures for relatively minor concerns related to aging and desired cosmetic improvement, the severity of these complications can be extremely disturbing to patient and physician alike.
The most common early clinical findings associated with vascular compromise are pain, skin blanching, livedo reticularis, slow capillary refill, and red-blue dusky discoloration. After several days, these initial symptoms are followed by the formation of blisters and tissue sloughing. The most important concept in management is truly avoidance via careful injection technique, however if it does occur, immediate recognition and treatment is vital. Initial treatments include immediate injection of hyaluronidase, warm compress, aspirin, and massage. Alternative, second line treatments include hyperbaric oxygen therapy as well as other vasodilating agents such as prostaglandin E1. Several revisions to protocols about how to treat intravascular occlusion have occurred within recent years as we find out more about this complication. The discussion of this evolution and current perspectives about management and treatment protocols will be discussed in greater detail later in this chapter.
Vasculature of the Face
A comprehensive knowledge of facial arterial anatomy is crucial for any physician practicing dermal filler injections. The main arterial blood supply to the face originates from the bilateral external carotid arteries. The facial artery and superficial temporal arteries arise directly from the external carotid artery, while the transverse facial artery and infraorbital arteries arise from branches of the external carotid artery (the superficial temporal artery and maxillary artery, respectively). One exception is the ophthalmic artery, which supplies the forehead, as it originates from the internal carotid artery. For the purposes of this discussion, we will focus on the facial artery. There have been several cadaveric studies regarding the course and variations of the facial artery. Though these studies report minor variations between them, they all agree with the fact that the facial artery has many variations in origin and branching pattern.1 In general, the facial artery, after branching from the external carotid artery, courses superiorly and crosses the mandible approximately 1 cm anterior the insertion of the masseter muscle. It continues on from there into the nasolabial fold deep to the perioral musculature before terminating as the angular artery approximately 0.5 cm from the nasal ala.
The anatomy of the facial artery and its branches make it particularly vulnerable to vascular complications from filler injection. It has been noted in cadaveric studies that the external and internal diameters and thickness of the artery gradually decreases from the inferior border of the mandible to the point bifurcation of the lateral nasal branch. Subsequent branches from the main branch of the facial artery have much smaller diameter than those of the main artery. Phumyoo et al showed the diameter of the artery to be 1.9 mm at the nasolabial fold, with a depth of 11.6 mm of the artery from the skin. The facial artery has also been noted to be more tortuous than the other arteries supplying the face, and this appears to increase with age.4
Danger Zones
Distinct “danger zones”, or areas with increased risk for vascular compromise with filler injection, have been identified as the glabella/brow, temporal region, perioral region, nasolabial fold, nose, and infraorbital regions. For example, the glabella and brow pose a risk primarily due to the arterial anastomosis of the supraorbital, supratrochlear, dorsal nasal, and angular arteries. These arteries become quite superficial after exiting the orbit and can closely approximate rhytids. Because of this rich network of superficially located vessels, intravascular cannulation of filler can lead to retrograde travel of material to the ophthalmic artery.4-6 Each of these subsites have their own unique reasons for increased risk. Here he will discuss in greater detail each of the danger zones of the face with regards to filler injection. Techniques for maximizing safety will be discussed in a later section of this document.
Brow/Glabella
Rhytids in the brow and glabellar region form primarily as a result of the contraction of the corrugators and the procerus muscles. The corrugators course superolateral, leading to vertical rhytids, or “eleven lines” over time, while the action of the procerus creates horizontal furrows between the eyes. The brown and glabellar region poses a unique danger due to the supratrochlear and supraorbital arteries, which are both branches of the ophthalmic artery. The supratrochlear artery exits the orbit approximately 17 to 22 millimeters lateral to midline, and either pierces or course superficially to the corrugator muscle. It initially runs deep to both the orbicularis and frontalis muscles, but at about 15 to 25 mm superior to the orbital rim it pierces both of these muscles to enter the subcutaneous plane. Anatomic studies have demonstrated variability in the location of the artery at the level of the brow, with some showing it running vertically within 3 mm of, or directly in line with, the medial canthus. An additional study showed the artery within the actual glabellar rhytid approximately 50% of the time.7-8
Not only does the supratrochlear artery pose a risk at the glabella, but the supraorbital artery also courses nearby. It exits the superior orbit at the supraorbital notch, approximately 32 mm lateral to the midline. It generally runs within a vertical line from the medial limbus of the eye. Most anatomic studies show that it regularly pierces the frontalis approximately 20 to 40 mm superior to the orbital rim, and enters the subcutaneous plane between 40 and 60 mm above the orbital rim.9 However, one study demonstrated vertical branches from the supraorbital artery in the subcutaneous tissue as low at 15 to 20 mm superior to the rim.10 Overall, this area poses a danger because the vasculature transitions from deep to intramuscular and subcutaneous quickly, and there are subtle variations in location. The dorsal nasal artery and angular artery are also present in this region and pose a risk for intravascular injection. Given that both the supratrochlear and supraorbital arteries branch from the ophthalmic artery, intravascular injection can travel retrograde and cause blindness and tissue loss. Multiple literature reviews have described the glabella as the most common site of filler injection to lead to loss of vision.11-13
Temporal Region
The temporal region poses a risk for filler injection due to the frontal branch of the superficial temporal artery. Along with the temporal branch of the facial nerve, the frontal branch of the superficial temporal artery runs within the temporoparietal fascia. A recent cadaveric study showed the frontal branch originating from the superficial temporal artery at an average of 17.2 mm anterior and 36.9 mm superior to the tip of the tragus. The frontal branch generally courses over the frontalis muscle 15.8 mm superior and 14.8 mm posterior to the arch of the brow. A line between these two described points generally outlines the most frequent course, though there are variations.14 Scheurer et al found the frontal branch of the superficial temporal artery running within the temporoparietal fascia about 2 cm above the zygomatic arch, before becoming entirely subcutaneous just superior to the brow. The artery anastomoses with the supraorbital arterial system in both the superficial and deep planes, offering another dangerous pathway for retrograde embolization to the eye.6 In fact, one cadaver study showed that injection of dye into the superficial temporal artery resulted in flow of the dye into the ipsilateral, and sometimes even bilateral, globes.15
In addition to the risk posed by the frontal branch of the superficial temporal artery, the middle temporal vein runs within the superficial temporal fat pad approximately 20 mm superior, and parallel to, the zygomatic arch. It is relatively large caliber, ranging from 2.0 to 9.1 mm (average of 5.1 mm). Given this, and its connection to the cavernous sinus, studies recommend injecting within a fingerbreadth of the zygomatic arch within the subperiosteal plane to avoid intravascular injection.16 While exceedingly rare, that have been reports of nonthrombotic pulmonary embolism from both fat and hyaluronic acid injection.17-18 Given these anatomic considerations, it is generally recommended that filler in the temporal region either be injected deep in the preperiosteal plane or just below the dermis in the superficial subcutaneous plane, in order to avoid intravascular compromise of both the superficial temporal artery and middle temporal vein.
Infraorbital Region
Filler injections to the midface have greatly reduced the popularity of alloplastic facial implants over the past decade. Despite the popularity of filler injection to this region, there are significant risks due to the underlying vascular anatomy. The infraorbital artery and nerve exit the infraorbital foramen, and inadvertent cannulation provides a route for orbital embolization and blindness. While this discussion focuses on vascular injury, it is also worth mentioning that injury to the infraorbital nerve can lead to hyperesthesia or hypoesthesia and pain. The literature is quite variable when it comes to the anatomical landmarks for the vertical plane of the infraorbital foramen.19-20 Studies have described it as approximating the first premolar, second premolar, and canine teeth. Even other studies have shown that some individuals have multiple foramina.21-22 Aziz et al found that 50% of the time the infraorbital foramen was in the same vertical plane as the supraorbital foramen.19 The average distance between the infraorbital foramen and inferior orbital rim is 6.3 to 10.9 mm, and the average distance of the infraorbital foramen from the midline of the face is 25.7 to 27.1 mm in men and 24.2 to 26.8 mm in women. In more general terms, the infraorbital foramen is typically located about one-third the distance between the medial and lateral canthi, and up to 11 mm below the infraorbital rim. For this reason, deep injections in this area are generally avoided within a fingerbreadths distance below the infraorbital rim in the vertical plane of the medial limbus.6
Perioral (lips/nasolabial fold)
As the desire for fuller lips grows, particularly with the popularity of social media, perioral filler injections are becoming increasingly common. There are subsites within the anatomical region, namely the upper lip, oral commissure, lower lip, and nasolabial fold, and each of these poses a unique risk due to underlying anatomy. Patients, particularly those that are young with baseline adequate volume, frequently request enhancement of the vermillion-cutaneous border. The superior labial artery generally resides several millimeters above the inferior border of the upper lip, posterior to the interface of the mucosa and the orbicularis oris muscle. Injection of filler intravascularly in this region can therefore cause tissue necrosis. The level of the oral commissure, the facial artery runs deep the risorius and zygomaticus major muscles approximately 12 to 15.5 mm lateral.26-27 There have been reported cases, however, of the artery coursing through individual zygomaticus bands.28 A reasonably clinical rule to abide by assumes that the facial artery and superior labial artery will reside within an area created by placing a thumb at the corner of the mouth.26
The biggest danger associated with filler injection the lower lip is the inferior labial artery. Anatomic studies have described the inferior labial artery running horizontally at the level of vermillion-cutaneous border, with vertical branches traversing the labiomental fold. After branching from the facial artery, the inferior labial artery enters the lower lip and courses between the mucosa and muscle, much like the superior labial artery of the upper lip. The inferior labial artery lies 6.4 to 7.1 mm from the anterior border of the lower lip at the vermillion-cutaneous border, 5.9 to 9.4 mm from the superior border, and 4.4 to 4.8 mm from the posterior border.29-32 The inferior labial artery is known to have numerous origins and variants, with some studies showing a common trunk with the superior labial artery, and some showing the absence of the artery entirely. Therefore, it is more important to consider the depth of the artery rather than the exact distance from the borders of the lip. For this reason, it is generally recommended to avoid injecting deeper than 3 mm at either the vermillion cutaneous border or within the dry vermillion.
While filler injection to the nasolabial fold can be a helpful tool in facial rejuvenation, the proximity of the facial artery and branches makes it a risk for vascular occlusion. The facial artery runs adjacent to the nasolabial fold after giving off the superior labial artery at the oral commissure. Cadaveric studies have shown a close relationship between the facial artery and the nasolabial fold, with its course being medial 42.9 percent of the time, lateral 23.2 percent of the time, and crossing the fold 33.9 percent of the time. The facial artery branches into the inferior alar artery and lateral nasal artery at the level of the nasal ala, before continuing on as the angular artery when present.33-34 There are many variations in the branching pattern of the facial artery and angular artery in this region, including the angular artery arising from the ophthalmic artery in a retrograde fashion.35 Similarly to injection of the lip, depth of injection is crucial to avoid vascular injury. A study by Lee et al. showed that in cadaveric specimens the facial artery was superficial to the mimetic muscles in at least one location between the alar base and the modiolus 85.2% of the time. Only 14.8% of cadavers had a facial artery that remained entirely deep to the facial muscles the entire distance to the alar base.36 The artery tends to become more superficial in the upper third of the nasolabial fold, and it is this area that is therefore more prone to injury from subcutaneous filler injection.5
Nose
Because of the immediate and dramatic results of the so-called “liquid rhinoplasty’, filler injection to the nose has become extremely popular. To review the layers of the nose, moving from superficial to deep, there is the epidermis, dermis, subcutaneous fat, muscle and fascia, areolar tissue, perichondrium/periosteum, and cartilage/bone.5,37 As was mentioned in the discussion of perioral vascular anatomy, the lateral nasal and angular arteries arise from the facial artery in numerous ways. On average, the facial artery is 3.2 mm lateral to the most lateral portion of the nasal ala.33-34 It gives rise to the inferior alar branch, which runs along the inferior margin of the nostril, as well as the lateral nasal artery, which courses in the subdermal plexus above the cephalic margin of the lower lateral cartilage. 36-39 After giving off these branches, the facial artery becomes the angular artery, anastomosing with the dorsal nasal arterial system, and travels vertically towards the medial canthus. A study by Toriumi et al. showed the presence of a subdermal vascular plexus, as well as a more robust arterial and venous nasal skin system superficial to the musculature. The areolar layer is devoid of vasculature apart from what he called the “deep” or “lateral nasal veins” that course superior to the lateral crura. Also, of import, the dorsal nasal artery, which is a terminal branch of the ophthalmic artery, courses over the nasal dorsum above the muscle to contribute to the subdermal plexus at the tip.39
The nose poses a unique risk for filler injection because not only are the vessels located superficially, but the vessels of the tip, dorsum, and sidewalls anastomose with the ophthalmic artery. Therefore, not only can intravascular injection lead to significant tissue necrosis, but it can result in blindness, as well. Due to both of these factors, it is recommended that injections of the tip and dorsum should be performed in the sub-SMAS, or preperichondrial/periosteal planes. All lateral injections should be performed greater than 3 mm cephalic to the alar groove and deep. Several reviews of facial danger zones have shown nasal filler injection to be the leading cause of tissue necrosis and second leading cause of visual loss.11-12
Overall, facial filler injections are increasingly popular and relatively safe procedures yielding immediate and impressive results. However, intravascular injection can result in catastrophic events- namely tissue necrosis and blindness. In-depth knowledge of vascular anatomy can help minimize the risk of injury. As described throughout this section, facial vascular anatomy can be extremely variable, which makes even the most experienced, knowledgeable practitioner at risk of complications. The most important factor for mitigation, beyond prevention, is to recognize complications early and manage them expeditiously. Management strategies will be discussed later on in this text.
Mechanisms of Vascular compromise
It is postulated that the mechanism of vascular occlusion from dermal fillers includes arterial, venous, and thromboembolic processes. Mechanical obstruction of an arterial vessel can result from either anterograde or retrograde flow of filler material.40 Once filler material enters an artery, the normal direction of blood flow may carry the filler material into progressively smaller diameter vessels, eventually resulting in obstruction. If this occurs in an area without adequate collateral blood supply, tissue ischemia and necrosis may occur.41 Retrograde flow of filler material may occur if the injection is completed with a high pressure and a rapid rate, such that the bolus is pushed proximally against the normal flow of blood. The filler embolism may reach a vessel branch point and become diverted to a different vessel and thus travel to distal sites supplied by this vessel. This mechanism could explain areas of ischemia caused by injections far from the original site of injection.41 Other proposed mechanisms include the activation of intravascular clotting systems or sclerosing reactions from the intravascular injection of the filler material, leading to intravascular thromboemboli, or the formation of an arterial thrombus secondary to stasis at the point of occlusion. 42 Compression of vessels by the external pressure of the dermal filler may also theoretically cause tissue ischemia, however experimental models have shown this to be a less likely cause.42,44 The ultimate goal of treatment should be to remove the injected material completely from the region as soon as possible.
Risk Factors:
The degree of tissue ischemia likely depends on a combination of factors, including anatomic site and location of injection, filler composition including particle size and cohesivity, and volume and rate of filler injection. Tissue ischemia has been reported from dermal filler injection sites throughout all regions of the face, although the highest risk areas for blindness continue to include the nose, glabella, and forehead.45
All types of filler have been associated with complications, including tissue ischemia and blindness. A recent study by Ortiz et al identified 3,782 complications from dermal fillers reported by the US FDA database from 1993-2004.46 Forty-four percent of complications implicated hyaluronic acid fillers, 40% involved poly-L-lactic acid fillers, 15% included calcium hydroxylapatite fillers, and <1% complications arose from polymethylmethacrylate fillers.46 Beleznay et al in 2015 reported that the most common filler type associated with blindness included autologous fat (48%), while other dermal filler agents made up the remaining 52%.47 Sorensen et al reported that blindness was associated with hyaluronic acid filler (70%), followed by autologous fat (11.7%), and calcium hydroxyapatite (11.7%) in a review of 60 new blindness cases from 2015-2018.45 These differences may reflect the increasing popularity of hyaluronic acid fillers over the past decade as compared to other types of fillers, estimated to compose 70% of the soft tissue injections presently performed in the United States.48
Interestingly, prior surgical interventions performed at sites of dermal filler application, including prior filler placement, and subsequent scar formation may distort the local anatomy and increase the risk of intravascular occlusion events. Theoretically as commented by DeLorenzi, a patient with many filler procedures in the past has a scar like environment and the needle or cannula use for injection would subsequently create a tunnel like area and if through a vessel within this tunnel can remain open temporarily and filler could potentially flow into the vessel.42 As the number of aesthetic procedures increases it is highly important to consider a patient’s past injection and surgical history at the site prior to injecting. It is important for the injector to recognize the risk factors present in each case and minimize controllable factors as much as possible to maximize safety. Above is an in-depth review of anatomy to understand for safety, and in a following section we will review potential safer practices when injecting.
Hyaluronic Acid and Hyaluronidase
Hyaluronic acid (HA) fillers are composed of hyaluronic acid, a biopolymer composed of repeating units of disaccharides, which belongs to a group of substances called mucopolysaccharides, which belong to the glycosaminoglycan (GAG) family. Chemical modification of HA is required to improve its mechanical properties in order to lift and fill wrinkles in the skin. This chemical modification differs between various HA fillers to enhance its particular properties designed to achieve differing clinical outcomes. The differences between the fillers lies within the cross-linking and concentration of the gel.49
Hyaluronidase is a soluble protein enzyme that catalyzes the degradation of HA by splitting the beta1 to 4 linkage, a glycosidic bond. It is manufactured in different formulations and derived from different sources (table 1), either purified bovine or ovine testicular hyaluronidase, or recombinant human DNA. In other countries, hyaluronidase is compounded and thus may have more variation in its preparation but achieves higher concentrations.42 Hyaluronidase can be inhibited by blood, and in vivo hyaluronidase is rapidly degraded.42 There exists a risk of allergic potential or sensitivity to hyaluronidase in individuals with Type 1 hypersensitivity reactions to insect stings, as bee or wasp venom contains hyaluronidase [50], although a review of dermatology literature did not demonstrate any cases of anaphylaxis or severe allergy when injected.51 This risk is thought to be higher with the purified bovine or ovine formulations, and potentially minimized by using the human recombinant formulation (hylenex). In a non-urgent situation, one can do an intradermal wheal in the arm and monitor for 20 minutes to see if a reaction occurs. However, in urgent situations, intradermal testing may not be practical, though the risks should be discussed with the patient .52 Providers are advised to have epinephrine readily available if this were to occur.
Table 1: Hyaluronidase Product Available on the Market
| Product | Origin | Manufacturer | Country | Units per vial |
| Hylenex | Human recombinant | Halozayme Therapeutics Inc | USA (FDA approved) | 150U/mL |
| Vitrase | Ovine testicular | Ista Pharmaceuticals | USA (FDA approved) | 200U/mL |
| Amphadase | Bovine testicular | Amphastar Pharmaceuticals | USA (FDA approved) | 150U/mL |
| Hylase “Dessau” | Bovine testicular | Riemser Arzneimittel AG | Germany | 150,300, 1500U/mL |
| Hyalase Powder | Not specified | CP Pharmaceuticals Ltd | United Kingdom | 1500u/mL |
| Compounded | Local compounding pharmacies | Not available in the USA | 1500u/mL |
In cases of accidental intravascular injection, hyaluronidase is used to break down the longer viscous and cohesive HA chain into smaller pieces. This results in a decrease in viscosity so the HA degradation products can pass through the vessel and relieve the vascular obstruction.42 One in vitro study by Kim et al suggested that biphasic fillers (Restylane) may have more resistance to hyaluronidase than monophasic fillers (Juvaderm).53 However, in vitro studies by DeLorenzi have suggested that the biphasic fillers may provide more surface area for the enzymatic degradation of hyaluronidase, and thus result in faster response to hyaluronidase. Additional studies are required to further characterize the optimal dosing of hyaluronidase for degradation of each particular filler, especially as more dermal filler formulations become available on the market. Nevertheless, when faced with soft tissue ischemic events, high dose protocols of hyaluronidase may be more relevant as will be discussed below.
Cadaveric models have shown that diffusion of hyaluronidase from surrounding tissue may be sufficient to achieve intravascular HA degradation (300U in 2mL NS, cadaveric model).42 Thus, direct injection of hyaluronidase into an occluded vessel is not required for degradation of HA filler. A rabbit ear model of intravascular occlusion clearly showed that hyaluronidase administration to surrounding soft tissues within four hours was more effective in preventing ischemic necrosis of tissue after intra-arterial HA embolism as compared to 24 hours or untreated controls. 43,53 Since it is nearly impossible to localize exactly which vessel has been occluded with filler in the outpatient setting, it is imperative to assume that all vessels within the zone of ischemia are obstructed and utilize hyaluronidase accordingly.42 In vivo, hyaluronidase is partially deactivated by natural agents, diluted by edema, and diffuses away from the region of interest.42 These local factors essentially reduce hyaluronidase action in vivo, suggesting higher dose protocols may be more effective in cases of intravascular occlusion.42
Treatment Algorithms
Intravascular occlusions are rare events, and thus recommendations for prevention and management are based primarily on expert opinion and consensus reports. It is important to recognize that the field is evolving and treatment protocols are becoming better understood, however a great deal of heterogeneity still exists between the protocol recommendations. Herein, we will include a discussion of protocols for the treatment of soft tissue ischemia and blindness due to intravascular dermal filler incidents. It is important for the reader to understand the salient points and be prepared with a plan in the unlikely event that a complication arises.
Prevention
There are various strategies that have been proposed to decrease the risk of inadvertent intra-arterial embolization of dermal filler products. Most importantly, the injector should have a thorough knowledge of the facial anatomy, vasculature, and “danger zones” reviewed extensively above.
The International consensus group by Goodman et al convened to specify recommendations focusing on the consenting process, prevention, and early management of visual impairment related to intravascular HA filler injection, which are also applicable to tissue related ischemic events, reviewed in below section.54 This group recommended various techniques aimed at preventing intravascular injection of dermal filler. Each injection should be completed slowly with low extrusion pressure, and with small aliquots of product <0.1cc. DeLorenzi (2019) differentiates low volume events (<0.1 mL of HA filler) versus high volume events (>0.1 mL of HA filler).42 While low volume events are the most common types of accidental intravascular events encountered by experienced users, high volume events are most often associated with worse prognoses and injuries such as vision loss, hearing loss, or even stroke. “Low and Slow” should be a guiding principle to all providers utilizing dermal filler agents, especially in high risk areas.42
Cannulas and needles have both been reported in intra-vascular events. Cannulas have been considered safer in the brow, lateral and anterior neck, but not considered safer for nasal injections. Cadaveric models suggest that cannulas result in a more precise injection of material relative to needles and use of needles resulted in distribution of material to more superficial layers than intended.55 However, smaller gauge cannulas (< 25g) have been considered to behave like a needle in its ability to pierce vessels. Thus, cannulas larger than 25g are preferred .55 The use of local anesthetic with epinephrine at cannula entry points should be considered, as local constriction of blood vessels could minimize intra-vascular injection risk. The needle should be moved within the chosen plane in small amplitude movements, and the direction of injections should always occur away from the eye. These strategies were concordant in another consensus statement by Cohen et al 2019, which discussed strategies to minimize soft tissue ischemia specifically.56 Notably, use of lidocaine throughout the treatment area is not recommended as this strategy may change the ability to detect blanching or other early signs of ischemia.
The most controversial recommendation among the consensus statements reviewed involved the discussion regarding aspiration prior to soft tissue dermal injection. The consensus by Goodman et al advise against aspiration in the prevention of blindness from intravascular injections, whereas Urdiales et al advocate for aspiration to prevent soft tissue ischemia and blindness.54, 57 Goodman et al discussed that there is currently no evidence to support aspiration as a safety measure, and that it is an unreliable and an impractical technique. Aspiration results are influenced by needle diameter, length, properties of the fillers injected, whether the needle is empty or contains filler in the lumen, the blood pressure, and the degree of negative pressure and time it is maintained to assess flashback.54 In vitro studies have shown a high false-negative rate, and a low true positive rate ranging from 33-53% of attempts.58 Goodman et al also argue that a negative aspiration may risk giving the injector a false sense of security to proceed with a risky injection of higher volume, while the positive aspiration is only relevant for the instant that the needle is in that position. Rather, the Goodman consensus proposes an alternative to aspiration – inject the product slowly and through a constantly moving needle, a technique with which we agree. This technique has the potential to minimize the amount of material delivered to any vessel encountered, and thus any among would be well below the threshold necessary to cause problems. In cases where a larger bolus is desired, the recommendation is to complete many oscillating movements of “micro-boluses” in the desired plane. All above recommendations are represented in table 2 to remind the reader of potential preventative strategies.
Table 2: Strategies for possible prevention of intravascular injection
| Understand the safest depth of injection in any given area |
| Inject slowly with low extrusion pressure |
| Inject small boluses <0.1cc in small aliquots43 |
| “Low and Slow” is good motto to repeat to re-iterate above principles- low volumes and slow injection pressure |
| Cannulas > 25g may have some safer qualities in some areas 55 |
| Consider directing cannula or needle perpendicular to the axis of a known vessel to minimize traveling into and along course of vessel |
| Continuous movement with injection |
| Inject away from the eye in high risk areas |
| No present evidence to fully support aspiration as safety measure |
| Be aware of scars in area (traumatic, surgical) or even if patient had many previous cosmetic injections (can create tunnel like area and flow into artery) 43 |
55. Pavicic, T., et al., Arterial Wall Penetration Forces in Needles versus Cannulas. Plast Reconstr Surg. 2019 Mar;143(3):504e-512e.
Treatment
The ultimate goal of these protocols is to restore the arterial circulation to the affected area to prevent soft tissue ischemia and resulting sequelae (i.e. tissue necrosis, blindness, stroke).42
These strategies have changed subtly over time; however, all utilize the same basic principles. Namely, to relieve the arterial obstruction via mechanical and enzymatic means and to augment blood flow in dermal arterioles via the use of topical and systemic agents if indicated. Below is text to describe some of these recommendations and highlight what we perceive to be the most important in the protocols up to date. Also provided is a table to contrast and compare the more pertinent literature of treatment recommendations, including consensus recommendations recently created for the use of calcium hydroxylapatite, a non-hyaluronic acid filler (table 3).
Table 3: Treatments of Tissue Ischemia from Accidental Intra-arterial Injection of HA based Dermal Filler
| DeLorenzi 2014. Tissue Ischemia.41 | Cohen, DeLorenzi 2015. Tissue Ischemia.56 | Urdiales consensus 2018. Tissue Ischemia and Blindness. 57 | DeLorenzi. 2019. High Dose protocol 43 | Van Loghem, et al. 2020. Managing intravascular complications with calcium hydroxylapatite.64 | |
| Prevention of Intra-arterial Injection- Technique | Not discussed | Not discussed | Recommend for aspiration prior to injection Low volumes and serial injections in high risk areas Treat one side at a time Pinch/tent the skin to provide more space superficial to the branches of the main arteries Manual occlusion of the origin of the supratrochlear vessels with the non-dominant finger Blunt cannulas may reduce but not eliminate risk | Aliquots of <0.1cc per pass in any 1 area of the face Inject under low pressure Avoid scarred areas, or treat as high-risk area | Knowledge of anatomy and high-risk areas Discussion with patient regarding prior procedures and surgeries that may alter anatomy. Low volumes, slow injection force, minimal product <0.1mL Aspiration is unreliable. Absence of flash back should not be reassuring. 22-25g blunt tipped cannula preferred in high risk zones. |
| Hyaluronidase (HYAL) | 300-600 IU into ischemic tissues Massage toward site “May be repeated at higher doses until desired effect” | “Use significant amount of hyaluronidase in area of necrosis” Start at minimum 200U HYAL Can dilute in either lidocaine or saline 1 injection for every 3-4 cm affected skin. Repeat every 60 mins for 3-4 cycles until improvement noted | High doses recommended (200-300U/1-2mL) along entire ischemic area Repeat hourly until clinical resolution achieved Doses up to 1500U may be required | High doses depending on quantity of tissue affected. 3cc of (150 u/cc) per half an upper lip. Repeat hourly Implement within 3 days of ischemic event | 600U for every 0.1cc of CaHA in the affected area. Assess hourly. Additional HYAL as needed every 2 hours. Repeat 3-4 times. |
| Massage | Warm compress and vigorous massage to help vasodilator, break up embolic material, and open up collateral vessels | Warm compress and massage vigorously. Warm compress for 5-10 mins every 30-60 mins | Warm gauze and massage | None | Warm compress |
| Topical Nitropaste | 1-2cm under occlusion at site of compromise Monitor VS Caution: hypotension | Immediately to affected areas 2-3 times daily, as tolerated Apply while lying down | Topical Nitro (1-2%) 2-3times per day Can use nitroglycerin SL tabs (no dose, frequency specified) | None | No clear recommendation due to conflicting data |
| Aspirin | 72mg po daily Duration not specified | 2x 325mg po ASA daily Antacid to avoid ASA-associated gastritis 1 wk course, but depends on clinical scenario | 500 mg q 8hrs for 24-48 hours might be helpful | 81mg daily for 7 days | Give 625mg ASA once acutely, then 75mg daily 3-5 days Pentoxifylline 400 TID may accelerate healing |
| Hyperbaric oxygen | “May help reduce the size of necrosis” | “Experience is limited, it is hard to assess its true value fully” | Mentioned, no recommendation for or against | None | |
| Prostaglandin E1 injection | “Usually administered under observation” | Mentioned, no recommendation for or against | Mentioned, no recommendation for or against | None | No clear recommendation |
| Sildenafil | Not discussed | Mentioned, no recommendation for or against | Mentioned, no recommendation for or against 1 daily for 3-5 days | None | May be used |
| Prednisone | Not discussed | Not discussed | Mentioned, no recommendation for or against 20-40mg daily, 3-5 days | None | 50mg daily with taper ever other day. Max 7 days. |
| LMWH | “Risk of bleeding, monitor coagulation profile” | Mentioned, no recommendation for or against | Mentioned, no recommendation for or against | None | Can be used within 4 hours of event |
| Follow up | Not discussed | Follow patients daily If improvement, NTG can be stopped If no improvement, repeat hyaluronidase, ASA, NTG daily for 2-3 days | Not discussed | Not discussed | Not discussed |
| Wound Care | Not discussed | Ointment Daily dressing changes Debridement as necessary General supportive care Monitor for infection | Not discussed | Not discussed | Consider PRP weekly Red light therapy 640nm with LED source Microneedling q 4wks. Gentle debridement of necrotic tissue and antibiotics Ointment and open dressings. |
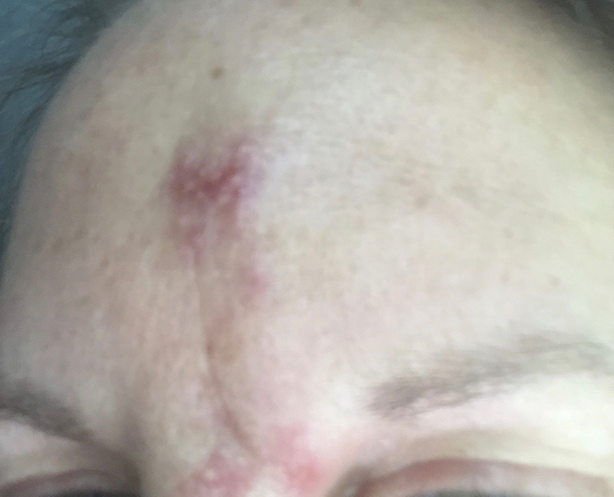
The injector should be able to readily identify the clinical signs and symptoms of a vascular occlusion event in order to minimize time to treatment (table 4). It is important to always be watching the skin in the area of injection and the surrounding areas while placing the filler substance because the first sign of intravascular occlusion is only a brief blanching. If seen, it is important not to ignore and to stop to assess for several minutes. Do not ignore any blanching you may see as nothing. If within minutes the area changes further to potential swelling, grayness, even sometimes increased erythema as the tissue is beginning to strangulate- the protocol should already be initiated by the injector and their assistants. Further, if you do not catch the signs immediately, and presented with a later sign of ischemic such as edema and blistering seen in figure 1, there is still opportunity to employ treatment and reverse potential scarring. A patient presenting hours later even within 72 hours, there is still opportunity to start high-dose hyaluronidase protocols to save ischemic cutaneous tissue. 43
Table 4: Clinical Signs and Symptoms of Potential Tissue Ischemia
| Signs and Symptoms of Tissue Ischemia | Onset Estimate |
| Blanching | Immediate, will be transient |
| Dusky Tissue, Edema, Decrease Capillary Refill of Tissue, Pain | Minutes to Hours |
| Livedo Reticularis | Hours to Days |
| Blistering | Day 3 and after |
| Crusting, Necrosis | Day 5 or after |
| Secondary Intention Healing or Scarring | Week 6 |
The use of topical agents, including topical nitroglycerin has become increasingly more controversial. Nitroglycerin is a known vasodilator active on arteries and veins and has been used off-label in cutaneous applications, including use in cutaneous graft and flap survival, and more recently in cases of suspected tissue necrosis due to intravascular occlusion. It has been traditionally believed that applying nitroglycerin paste to affected areas may improve perfusion, as demonstrated experimentally by Schonberger and colleagues. This ability of topical nitroglycerin to induce a local vasodilatory response in small- or medium-caliber dermal vessels is the basis for its use by physicians in the setting of filler-induced ischemia.59 However, recent studies have called its application for the use of tissue ischemia into question. Hwang et al suggested that use of nitroglycerin paste on a rabbit ear model may in fact worsen ischemia, with dilation of vessels and further propagation of the product into the smaller arterioles and capillaries.60 The adverse systemic effects of nitroglycerin, including hypotension and dizziness may also be a limiting factor for its use. A recent review of the literature by Carley et al reported 7 of 66 cases of tissue necrosis used topical nitroglycerin as a part of the treatment algorithm. 6 of 7 cases were successful in halting impending necrosis, though 4 of the 6 successful cases also reported use of hyaluronidase in conjunction.61 Thus, the evidence surrounding the use of topical nitroglycerin remains inconclusive though its use has still been reported in recent consensus statements.56,57
The most critical factor in treatment is widely accepted to be the use of hyaluronidase for treatment of filler embolism. Recently, DeLorenzi’s 2019 high dose pulsed hyaluronidase protocol provides an interesting update to the currently accepted and multi-modal hyaluronidase approaches. He proposes that dosing of hyaluronidase should depend on the quantity of tissues adversely affected, rather than a “one dose fits all” approach.42 The underlying assumption of this treatment strategy is that 1) all vessels within the zone of ischemia should be presumed to be obstructed and 2) sufficient hyaluronidase should be used in the entire zone to promote complete hydrolysis of the filler. Partial hydrolysis is an inadequate treatment endpoint since partial breakdown products may still be carried downstream and result in new distal areas of ischemia. Since hyaluronidase must diffuse across the intact arterial wall into the vessel lumen to dissolve the filler, sufficient concentrations of hyaluronidase should be used to achieve this endpoint. As discussed previously, hyaluronidase is metabolized by the body and continuously deactivated, is diluted in the setting of tissue edema, and readily diffuses in vivo. Since these factors serve to reduce hyaluronidase action, DeLorenzi proposes that hyaluronidase concentrations should be continuously maximized with an hourly dosing schedule based on clinical experience with this method.42 The high dose hyaluronidase protocol thus involves using a stepwise hyaluronidase dose per ischemic anatomic volume involved and repeating this dose every hour until the endpoint is achieved which is capillary refill indicating perfusion of the effected tissue. The proposed hyaluronidase dose is approximately 500U of hyaluronidase to each subunit area seemingly undergoing ischemic change. While the hour re-dosing schedule is based on the author’s clinical experience, further studies are needed to better elucidate the frequency of re-dosing to achieve optimal reversal of ischemic signs and symptoms.
Please review the table 3 and see the differing treatment protocols from various authors. Be mindful of what you are looking for suspected intravascular injection and have a plan. Be ready in your office with high doses of hyaluronidase (minimum suggested by authors is 1000-1500U) and have access to get more within the hour- local colleagues, pharmacies, etc. If unable to obtain more hyaluronidase quickly, it would be wise to stock many more units in your clinic. Have aspirin 325mg on hand and have the ability to create a warm compress quickly. When injecting the hyaluronidase and flooding the area, it will be painful for the patient, letting them know that pain and burning during this phase is normal but restoration of capillary refill and cessation of pain are the goals of the treatment protocol. The tissue will be pink, ecchymosis for all the repeated injections so it may be confusing to tell when you have completed the course for hyaluronidase- ideally keep capillary refill as an endpoint, pressing on the tissue to see if has been restores. Please continue to inject serially (hourly as recommended by the most recent protocol) until this endpoint it met.43
Blindness
Blindness and ischemic stroke are also feared complications of intravascular occlusion from filler injections. The actual rate of these complications is not known as many are not reported in the literature and therefore, treatment protocols are even more sparse but evolving. A recent updated review article by Sorensen and Council where there were 98 new cases of blindness from 2015-2018, hyaluronic acid accounted for 70.0% of the cases, followed by autologous fat (11.7%), and calcium hydroxyapatite (11.7%).47 Previously autologous fat injections were the highest number of cases reported associated with blindness. Still the highest risk areas associated were from the nose (55%), glabella (35%), and forehead (18.3%) likely from the higher amount of anastomosis in these areas between the internal and external carotid systems.47 Blindness is likely occlusion of the ophthalmic end arteries including the central retinal artery (CRA) from anastomosis from better known connections in the supraorbital, supratrochlear, dorsal nasal arteries but potential for flow into the eye circulation can occur from the zygomaticofacial (malar eminence) and zygomaticotemporal arteries (temporal fossa) or even the angular branch of the facial artery, a very common area of injection of filler substances.54
Furthermore, the rate of concomitant stroke at 16.7% of cases reported is significant and something all injectors should be aware of. There are several symptoms a clinician should monitor for that may indicate impending ocular or cerebral damage. In this review article of 98 patients is important to highlight the numbers which were eye pain (42.3%), nausea or vomiting (20.0%), headache (16.7%). Signs associated with these cases were ophthalmoplegia (43.3%), ptosis (40.0%), skin necrosis (31.7%), and acute ischemic stroke (16.7%). Similar to the factors associated with tissue ischemia, the degree of visual impairment depends on the vessels affected, the filler properties, and quantity and rate of filler injected.62 Blindness has been reported with both needle and cannula use.
As for treatment, again recommended options are far and few between but a good academic debate is occurring in the literature to try to determine which may help and which may be ultimately dangerous. Differing form ischemic events of soft tissue on the face, retinal ischemia occurs quickly within 90 minutes or quite possibly much shorter62, therefore the speed to administer potential treatments is of the utmost importance. Treatments reported included a combination of many modalities most of which focuses on the immediate injection of hyaluronidase. Hyaluronidase has been reported to be attempted in several different manners including injecting subcutaneous at area of injection, injecting the supratrochlear, supraorbital, and/or infraorbital foramen area, injecting retrobulbar, and lastly attempted injection of hyaluronidase intra-arterial. Overall, there are only case reports of some limited success with the use of supratrochlear, supraorbital, dorsal nasal and retrobulbar injections of hyaluronidase.63,54 In the most recent consensus guidelines specially for vision loss after a hyaluronic acid filler injection, Goodman and colleagues recommend in the case of visual impairment having a plan in place for transfer of care to a provider with advanced training on evaluating and treating with hyaluronidase in or around the eye. Therefore, it is important to have these conversations with those in your community and have a plan on record for your office. If not delaying the transfer of care, one can consider high dose hyaluronidase injected where the filler was placed and any other areas which show signals of impending skin necrosis, they recommend using 1500 units of hyaluronidase for these injections.54 They further comment that one could inject a high dose of hyaluronidase at the supraorbital margin at the supratrochlear artery (approximately 14mm from midline beneath the medial brow creases) and possibly at the supraorbital notch or foramen (approximately 25mm from midline) or other branches of the ophthalmic arterial system only if the injector is experienced. They believe that retrobulbar injection of hyaluronidase should only be attempted with advanced trained personnel. Also, if not delaying a potential transfer, one could document exam findings including time of event, visual deficit, visual loss (ie: unable to read x,y,z or holding fingers up), pupillary reactions, extraocular eye movements, and possible associated signs of ischemic stroke (speech, arm/hand movement, etc). One could take photos/videos to monitor for progression or improvement or to communicate with another physician, but again not to delay transfer in these activities.54 Please refer to table 5 for basic reminders of how to manage this potential complication in your own setting, as these recommendations are in its infancy remember they will change so up to date knowledge of the literature will be necessary.
Table 5: Considerations if ischemic ocular or neurologic complications are suspected
| Goodman Consensus. 2020. Blindness.54 | Van Loghem, et al. 2020. Managing intravascular complications with calcium hydroxylapatite. 64 | |
| Prevention techniques | Recommend against aspiration Inject product slowly through constantly moving needle Inject slowly with low extrusion pressure Small aliquots <0.1ccCannula safer for brow, lateral/anterior cheek; not safer for nasal injection Consider local anesthetic with epi at entry points Move needle in plane chosenInject away from the eye | Knowledge of anatomy and high-risk areas Discussion with patient regarding prior procedures and surgeries that may alter anatomy. Low volumes, slow injection force, minimal product <0.1mLAspiration is unreliable. Absence of flash back should not be reassuring. 22-25g blunt tipped cannula preferred in high risk zones. |
| Acute Considerations | Early recognition is vital; ischemia is often irreversible Initiate transfer to a facility with expertise and tools to evaluate blindness and stroke (plan should already be established) Document signs/symptoms indicating ocular or neurologic ischemiaConsider high dose HYAL in areas injected and areas showing signs of cutaneous ischemia Consider high dose HYAL in supraorbital or supratrochlear arteries | Ocular (eyeball) massage for blindness and rebreathe in paper bag to increase CO2 levels Give 625mg ASA in acute setting Pentoxifylline 600 TID Timolol 0.5%, brinzolamide, acetazolamide eye drops Immediate transport to ophthalmologist.Hospitalization and possible IV PGE1, dexamethasone, hyperbaric oxygen therapy |
Summary
As more dermal filler products continue to be introduced to the market, and the number of procedures continues to increase in popularity, the number of associated complications is not surprisingly projected to increase proportionally. The clinician performing these procedures should be well trained in facial anatomy prior to injecting and maintain vigilance when assessing for signs and symptoms of local complications such as vascular compromise. Preventative measures and treatment protocols are continuously updated to optimize patient outcomes and safety. While many protocols and consensus statements presently exist in the literature, recent evidence points to superior clinical outcomes with high dose hyaluronidase protocols. Please have a plan with all members of your care team in your office to treat intravascular necrosis, including education of the front desk personnel who may answer the phone call to “I have pain and a weird bruise” to ensure the patient returns to your clinic immediately for evaluation. All must be on board to treat this potential complication from dermal filler injection.
References
- Phumyoo T et al. The Soft Tissue Landmarks to Avoid Injury to The Facial Artery During Filler and Neurotoxin Injection at the Nasolabial Region. The Journal of Craniofacial Surgery 2014; 25(5).
- DeLorenzi C. Complications of injectable fillers, part 2: vascular complications. Aesthet Surg J. 2014 May 1;34(4):584-600.
- Lee SH et al. External and Internal Diameters of the Facial Artery Relevant to Intravascular Filler Injection. Plast Reconst Surg 2018; 143: 2031.
- Soikkonen K. Three main arteries of the face and their tortuosity. Br J Oral Maxillofac Surg 1991; 29(6):395-8.
- Scheurer JF et al. Facial Danger Zones: Techniques to Maximize Safety during Soft-Tissue Filler Injections. Plast Reconst Surg 2016; 139:1103.
- Scheurer JF et al. Anatomy of the Facial Danger Zones: Maximizing Safety during Soft-Tissue Filler Injections. Plast Reconst Surg 2017; 139:50.
- Shumrick KA, Smith TL. The anatomic basis for the design of forehead flaps in nasal reconstruction. Arch Otolaryngol Head Neck Surg. 1992;118:373–379.
- Webster RC, Gaunt JM, Hamdan US, Fuleihan NS, Giandello PR, Smith RC. Supraorbital and supratrochlear notches and foramina: Anatomical variations and surgical relevance. Laryngoscope 1986;96:311–315.
- Erdogmus S, Govsa F. Anatomy of the supraorbital region and the evaluation of it for the reconstruction of facial defects. J Craniofac Surg. 2007;18:104–112.
- Kleintjes WG. Forehead anatomy: Arterial variations and venous link of the midline forehead flap. J Plast Reconstr Aesthet Surg. 2007;60:593–606
- Ozturk CN, Li Y, Tung R, Parker L, Piliang MP, Zins JE. Complications following injection of soft-tissue fillers. Aesthet Surg J. 2013;33:862–877.
- Li X, Du L, Lu JJ. A novel hypothesis of visual loss secondary to cosmetic facial filler injection. Ann Plast Surg. 2015;75:258–260.
- Park SW, Woo SJ, Park KH, Huh JW, Jung C, Kwon OK. Iatrogenic retinal artery occlusion caused by cosmetic facial filler injections. Am J Ophthalmol. 2012;154:653–662.e1.
- Lee JG, Yang HM, Hu KS, et al. Frontal branch of the superficial temporal artery: Anatomical study and clinical implications regarding injectable treatments. Surg Radiol Anat. 2015;37:61–68.
- Tansatit T, Moon HJ, Apinuntrum P, Phetudom T. Verification of embolic channel causing blindness following filler injection. Aesthetic Plast Surg. 2015;39:154–161.
- Jung W, Youn KH, Won SY, Park JT, Hu KS, Kim HJ. Clinical implications of the middle temporal vein with regard to temporal fossa augmentation. Dermatol Surg. 2014;40:618–623.
- Jiang X, Liu DL, Chen B. Middle temporal vein: A fatal hazard in injection cosmetic surgery for temple augmentation. JAMA Facial Plast Surg. 2014;16:227–229.
- Jang JG, Hong KS, Choi EY. A case of nonthrombotic pulmonary embolism after facial injection of hyaluronic acid in an illegal cosmetic procedure. Tuberc Respir Dis 2014;77:90–93.
- Aziz SR, Marchena JM, Puran A. Anatomic characteristics of the infraorbital foramen: A cadaver study. J Oral Maxillofac Surg. 2000;58:992–996.
- Raschke R, Hazani R, Yaremchuk MJ. Identifying a safe zone for midface augmentation using anatomic landmarks for the infraorbital foramen. Aesthet Surg J. 2013;33:13–18.
- Agthong S, Huanmanop T, Chentanez V. Anatomical variations of the supraorbital, infraorbital, and mental foramina related to gender and side. J Oral Maxillofac Surg. 2005;63:800–804.
- Aggarwal A, Kaur H, Gupta T, et al. Anatomical study of the infraorbital foramen: A basis for successful infraorbital nerve block. Clin Anat. 2015;28:753–760.
- Canan S, Asim OM, Okan B, Ozek C, Alper M. Anatomic variations of the infraorbital foramen. Ann Plast Surg. 1999;43:613–617.
- Cutright B, Quillopa N, Schubert W. An anthropometric analysis of the key foramina for maxillofacial surgery. J Oral Maxillofac Surg. 2003;61:354–357.
- Hwang SH, Kim SW, Park CS, Kim SW, Cho JH, Kang JM. Morphometric analysis of the infraorbital groove, canal, and foramen on three-dimensional reconstruction of computed tomography scans. Surg Radiol Anat. 2013;35:565–571.
- Lee SH, Gil YC, Choi YJ, Tansatit T, Kim HJ, Hu KS. Topographic anatomy of the superior labial artery for der-mal filler injection. Plast Reconstr Surg. 2015;135:445–450.
- Loukas M, Hullett J, Louis RG Jr, et al. A detailed observa-tion of variations of the facial artery, with emphasis on the superior labial artery. Surg Radiol Anat. 2006;28:316–324.37. Pinar YA, Bilge O, Govsa F. Anatomic study of the blood sup-ply of perioral region. Clin Anat. 2005;18:330–339
- Kwak HH, Hu KS, Youn KH, et al. Topographic relationship between the muscle bands of the zygomaticus major muscle and the facial artery. Surg Radiol Anat. 2006;28:477–480.
- Tansatit T, Apinuntrum P, Phetudom T. A typical pattern of the labial arteries with implication for lip augmentation with injectable fillers. Aesthetic Plast Surg. 2014;38:1083–1089.34.
- Al-Hoqail RA, Meguid EM. Anatomic dissection of the arte-rial supply of the lips: An anatomical and analytical approach. J Craniofac Surg. 2008;19:785–794.
- Edizer M, Mağden O, Tayfur V, Kiray A, Ergür I, Atabey A. Arterial anatomy of the lower lip: A cadaveric study. Plast Reconstr Surg. 2003;111:2176–2181.
- Pinar YA, Bilge O, Govsa F. Anatomic study of the blood sup-ply of perioral region. Clin Anat. 2005;18:330–339.
- Nakajima H, Imanishi N, Aiso S. Facial artery in the upper lip and nose: Anatomy and a clinical application. Plast Reconstr Surg. 2002;109:855–861; discussion 862–863.
- Yang HM, Lee JG, Hu KS, et al. New anatomical insights on the course and branching patterns of the facial artery: Clinical implications of injectable treatments to the nasolabial fold and nasojugal groove. Plast Reconstr Surg. 2014;133:1077–1082.
- Kim YS, Choi DY, Gil YC, Hu KS, Tansatit T, Kim HJ. The anatomical origin and course of the angular artery regarding its clinical implications. Dermatol Surg. 2014;40:1070–1076.
- Lee JG, Yang HM, Choi YJ, et al. Facial arterial depth and relationship with the facial musculature layer. Plast Reconstr Surg. 2015;135:437–444.
- Saban Y, Andretto Amodeo C, Hammou JC, Polselli R. An anatomical study of the nasal superficial musculoaponeu-rotic system: Surgical applications in rhinoplasty. Arch Facial Plast Surg. 2008;10:109–115.
- Rohrich RJ, Gunter JP, Friedman RM. Nasal tip blood supply: An anatomic study validating the safety of the transcolumel-lar incision in rhinoplasty. Plast Reconstr Surg. 1995;95:795–799; discussion 800–801.50. Saban Y, Andretto Amodeo C, Bouaziz D, Polselli R. Nasal arterial vasculature: Medical and surgical applications. Arch Facial Plast Surg. 2012;14:429–436
- Toriumi DM, Mueller RA, Grosch T, Bhattacharyya TK, Larrabee WF Jr. Vascular anatomy of the nose and the exter-nal rhinoplasty approach. Arch Otolaryngol Head Neck Surg. 1996;122:24–34
- Paap, M.K., et al., Examining the Role of Retrobulbar Hyaluronidase in Reversing Filler-Induced Blindness: A Systematic Review. Ophthalmic Plast Reconstr Surg, 2020. 36(3): p. 231-238.
- DeLorenzi, C., Complications of injectable fillers, part 2: vascular complications. Aesthet Surg J, 2014. 34(4): p. 584-600.
- DeLorenzi, C., Transarterial degradation of hyaluronic acid filler by hyaluronidase. Dermatol Surg, 2014. 40(8): p. 832-41.
- DeLorenzi, C., New High Dose Pulsed Hyaluronidase Protocol for Hyaluronic Acid Filler Vascular Adverse Events. Aesthet Surg J, 2017. 37(7): p. 814-825.
- Chang, S.H., et al., External Compression Versus Intravascular Injection: A Mechanistic Animal Model of Filler-Induced Tissue Ischemia. Ophthalmic Plast Reconstr Surg, 2016. 32(4): p. 261-6.
- Sorensen, E.P. and M.L. Council, Update in Soft-Tissue Filler-Associated Blindness. Dermatol Surg, 2020. 46(5): p. 671-677.
- Ortiz, A.E., et al., Analysis of U.S. Food and Drug Administration Data on Soft-Tissue Filler Complications. Dermatol Surg, 2020. 46(7): p. 958-961.
- Beleznay, K., et al., Avoiding and Treating Blindness From Fillers: A Review of the World Literature. Dermatol Surg, 2015. 41(10): p. 1097-117.
- Rohrich, R.J., E.L. Bartlett, and E. Dayan, Practical Approach and Safety of Hyaluronic Acid Fillers. Plastic and Reconstructive Surgery – Global Open, 2019. 7(6).
- Stocks, D., et al., Rheological evaluation of the physical properties of hyaluronic acid dermal fillers. J Drugs Dermatol, 2011. 10(9): p. 974-80.
- Lyall, D.A.M., et al., A sting in the tale: cross reaction hypersensitivity to hyaluronidase.
- Landau, M., Hyaluronidase Caveats in Treating Filler Complications. Dermatol Surg, 2015. 41 Suppl 1: p. S347-53.
- Kim, T.W., et al., Allergic reactions to hyaluronidase in pain management -A report of three cases. Korean journal of anesthesiology, 2011. 60(1): p. 57-59.
- Park, K.Y., H.K. Kim, and B.J. Kim, Comparative study of hyaluronic acid fillers by in vitro and in vivo testing. J Eur Acad Dermatol Venereol, 2014. 28(5): p. 565-8.
- Goodman, G.J., et al., A Consensus on Minimizing the Risk of Hyaluronic Acid Embolic Visual Loss and Suggestions for Immediate Bedside Management. Aesthet Surg J, 2020. 40(9): p. 1009-1021.
- Pavicic, T., et al., Arterial Wall Penetration Forces in Needles versus Cannulas. Plast Reconstr Surg. 2019 Mar;143(3):504e-512e.
- Cohen, J.L., et al., Treatment of Hyaluronic Acid Filler-Induc Treatment of Hyaluronic Acid Filler-Induced Impending Necrosis With Hyaluronidase: Consensus Recommendations. Aesthet Surg J. 2015 Sep;35(7):844-9.
- Urdiales-Gálvez, F., et al., Treatment of Soft Tissue Filler Complications: Expert Consensus Recommendations. Aesthetic Plast Surg, 2018. 42(2): p. 498-510.
- Van Loghem, J.A., J.J. Fouché, and J. Thuis, Sensitivity of aspiration as a safety test before injection of soft tissue fillers. J Cosmet Dermatol, 2018. 17(1): p. 39-46.
- Kleydman, K., J.L. Cohen, and E. Marmur, Nitroglycerin: a review of its use in the treatment of vascular occlusion after soft tissue augmentation. Dermatol Surg, 2012. 38(12): p. 1889-97.
- 60. Hwang, C.J., et al., Rethinking the Role of Nitroglycerin Ointment in Ischemic Vascular Filler Complications: An Animal Model with ICG Imaging. Ophthalmic Plast Reconstr Surg, 2016. 32(2): p. 118-22.
- Carley, S.K., C.N. Kraus, and J.L. Cohen, Nitroglycerin, or Not, When Treating Impending Filler Necrosis. Dermatologic Surgery, 2020. 46(1).
- DeLorenzi, C. Discussion: Assessing Retrobulbar Hyaluronidase as a Treatment for Filler-Induced Blindness in a Cadaver Model. Reconstr Surg. 2019 Aug;144(2):321-324.
- Chesnut C. Restoration of visual loss with retrobulbar hyaluronidase injection after hyaluronic acid filler. Dermatol Surg. 2018;44(3):435-37.
- van Loghem J, Funt D, Pavicic T, Goldie K, Yutskovskaya Y, Fabi S, Siebenga P, Thuis J, Hkeik J, Kadouch J, Prager W, Azib N, Casabona G, Dayan S, Bay Aguilera S, Snozzi P, Saeed P. Managing intravascular complications following treatment with calcium hydroxylapatite: An expert consensus. J Cosmet Dermatol. 2020 Mar 17. Epub ahead of print. PMID: 32185876.
