Disclosures
Dr. Werschler is a consultant for Suneva Medical, Inc, Ulthera, and Abbvie; has grant/research support from Allergan, Amgen, Dermavance, Galderma, Genentech, Kythera, Suneva, Ulthera, Janssen, Pfizer, and Boehringer Ingelheim; has received honoraria from Allergan, Merz, Suneva , Abbvie, Celgnene, Leo, Merz, Continueed Med and Prescribers choice; and has served on the speakers bureau for Allergan, Celgene, Leo, Merz, Ulthera, Xoft, Abbvie, Prescriber’s choice, Pfizer, and Eskata.
Funding Support
Editorial support for this article was provided By Ginny Vachon PhD and Cecelia Wall, MS from Principal Medvantage, LLC, Atlanta Georgia, USA. Funding for medical writing support was provided by Suneva Medical, Inc.
Abstract
Acne is extremely common and in a subset of affected individuals can leave scars that persist into adulthood, causing significant physical and psychological distress. This chapter provides an overview of acne scarring and how acne scars are treated, focusing on microneedling with and without platelet-rich plasma, laser treatments, and fillers (specifically Bellafill®, Suneva Medical, San Diego, CA) the only long-lasting filler FDA-approved for use in treating moderate to severe atrophic acne scars.
Overview of Acne Scars
Acne is extremely common, affecting 80% to 90% of adolescents and persisting into adulthood in 5% to 14% of cases. Although all areas of the body with high concentrations of pilosebaceous glands can be involved, the most common affected areas include the face, back, and chest. In a small proportion of affected individuals (up to 11%), acne scars form as a result of the severe inflammatory response, resulting in textural changes in the superficial and deep dermis that are reflected in the overlying epidermis. Additionally, subcutaneous tissues may be affected leaving surface depressions. Acne scars affect quality of life and are associated with significant physical and psychological distress not only in adolescents but also adults.
The pathogenesis of acne is multifactorial and includes increased sebum production, alteration of the sebum lipid quality, androgen activity, proliferation of Propionibacterium acnes within the follicle, and follicular hyperkeratinization. Such factors result in the stimulation of the infra-infundibular inflammatory process, follicular rupture, and the formation of perifollicular abscesses, all of which stimulate wound-healing processes. Wound healing occurs in 3 stages: inflammation, granulation tissue formation, and matrix remodeling.
Inflammation
The severity and duration of inflammation is related to the development of scarring. Inflammation causes the recruitment of several types of blood cells, including granulocytes, macrophages, neutrophils, lymphocytes, fibroblasts, and platelets, which act to ready the site for granulation tissue formation. In addition, inflammation may stimulate melanogenesis, which can contribute to the development of post-acne erythema and hyperpigmentation.
Granulation Tissue Formation
Damaged tissues are repaired and new capillaries form during the granulation tissue formation process. Over time, neutrophils are replaced by monocytes, which mature into macrophages and release growth factors that stimulate the migration and proliferation of fibroblasts. Fibroblasts begin producing collagen approximately 3 to 5 days after the wound, with type III collagen predominating early on and eventually shifting to approximately 80% type I collagen over time.
Matrix Remodeling
Matrix remodeling is the third step in the wound healing process, during which fibroblasts and keratinocytes produce a variety of enzymes, including that create the extracellular matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs. An imbalance in the ratio of tissue inhibitors of MMPs and MMPs can result in formation atrophic scars if there is a diminished deposition of collagen factors or hypertrophic scars if the healing response is exaggerated and forms a raised nodule of fibrotic tissue.
Types of Acne Scars
Atrophic acne scars are categorized as ice pick scars, boxcar/poxmark scars, and rolling boxcar scars (Table 1). Most patients have multiple types of atrophic acne scars, and it can be difficult to distinguish between types. Several classification systems and scales have been proposed, such as the Goodman and Baron qualitative and quantitative scales and the Echelle d’Evaluation Clinque des Cicatrices d’Acné (ECCA) scale.1-3
It is important to note that acne scars are different than surgical scars in that they do not improve over time. Instead, the collagen matrix is not normalized, and the compounding effects of volume loss with age and reduced skin elasticity make the acne scars more apparent.
Table 1. Overview of Atrophic Acne Scars.
| Scar Subtype | Description | Image |
| Ice Pick | Narrow (<2 mm) but deep epithelial tracts that typically form a “V” shapeExtend vertically to the deep dermis or subcutaneous tissue | 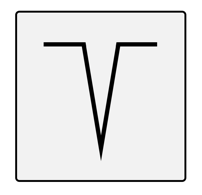 |
| Rolling Boxcar | Typically wider than 4-5 cmOccur due to dermal tethering of otherwise normal-appearing skin, typically forming an “M” shapeAbnormal fibrous anchoring of the dermis to the subcutis leads to superficial shallowing and a rolling appearance of the overlying skinEdges do not disappear when stretched | 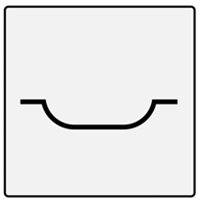 |
| Boxcar/Poxmark | Round or oval depressions with well-established vertical edges, typically forming a “U” shapeSimilar in appearance to varicella scarsMay be shallow (0.1-0.5 mm) or deep (≥0.5 mm)Typically 1.5-4.0 mm in diameterEdges disappear when stretched | 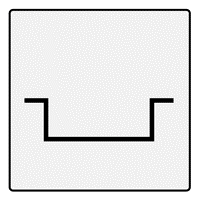 |
Hypertrophic and keloidal scars are associated with decreased collagenase activity and excess collagen deposition. Hypertrophic scars contain thick, hyalinized collagen bundles that reside within the area of the original site of injury, whereas keloids are reddish-purple papules and nodules that proliferate outside the borders of the original wound. Both hypertrophic and keloidal scars tend to occur mostly on the trunk and are more prevalent in individuals with darker skin. This chapter focuses on the treatment of atrophic acne scars.
Overview of Treatment
Treatment of acne scarring can be challenging, but new options are becoming available that have been shown to diminish atrophic scars. Many methods have been evaluated in the treatment of acne scars including topical preparations, chemical peels, focal treatment with trichloroacetic acid, dermabrasion, laser resurfacing, subcutaneous incision, punch excision/elevation, dermal grafting, fillers, fat transfer, implantation of autologous collagen, implantation of cultured autologous fibroblasts, and skin microneedling. This chapter will focus on microneedling, laser treatment, and filler use. The only approved permanent filler for the treatment of acne scars is Bellafill®, which will be a major focus of this chapter.
In general, the type of treatment depends on the type and severity of scars. Typically, the deepest scars are first treated with surgery. Atrophic scars may then be treated with microneedling with or without platelet-rich plasma. Finally, surface irregularities are addressed using laser treatment or filler.
Platelet-rich plasma (PRP) may be utilized as an adjunctive treatment with other modalities, including microneedling and laser treatments. PRP, which is rich in growth factors and cytokines (eg, transforming growth factor-ß, platelet-derived growth factor, vascular endothelial growth factor, and others), regulates and induces cell migration, attachment, proliferation, and differentiation, ultimately resulting the accumulation of extracellular matrix and improved acne scar appearance. PRP is generally considered to be safe with minimal side effects and few contraindications.
Microneedling
In microneedling, a needle is used to puncture the skin multiple types using a device such as a roller. Rollers are drum-shaped devices with fine, protruding stainless steel sterile needles that are 0.25-3 mm in length. The needles penetrate the skin, causing localized damage, minor bleeding, and microbruising. In addition, the punctures disrupt the collagen bundles in the superficial dermis that are responsible for scars. This disruption of collagen results in the induction of new collagen formation under the epidermis.
Histology sections after microneedling show a dramatic increase in collagen and elastin fibers with visible results within 6 weeks, although full effects may be visible after >3 months. Because the deposition of collagen is gradual, skin texture continues to improve over ~12 months. In a quantitative evaluation of histological changes after microneedling, a statistically significant increase in epidermal thickness with increased formation of dermal papillae was noted. In addition, the formation and deposition of type III and type I collagen and elastin were significantly increased by 3 months after treatment. Initially, collagen type III was predominant but gradually converted to collagen type I, resulting in improvement of acne scar appearance.4
In a prospective, single-center study of 31 Vietnamese patients with atrophic acne scars who received microneedle dermaroller treatment every week for 3 months, all patients showed improvement with a decrease in Goodman and Barron’s grade from 3.29 ± 0.59 at baseline to 1.77 ± 0.57 at 2 months after completion of therapy (P <0.05), including improvements in skin texture and hyperpigmentation. Overall, 83.3% of patients were satisfied after the completion of therapy.5
Generally, patients require 3 to 6 treatments spaced 1 to 4 weeks apart. Most patients achieve some improvement, although the degree of improvement varies. Responses appear to be best with rolling and boxcar scars, while icepick scars may have moderate response. Side effects are generally mild, including burning sensation, transient erythema and edema resolving within 1-2 days. Notably, dermarollers with larger needles (eg, 3 mm) may produce better results, but also are associated with longer downtime and larger risk of side effects and complications.4,6
PRP may be used in conjunction with microneedling to enhance the final clinical outcomes. However, clinical results have been mixed. For example, in a study of 50 patients aged 17 to 32 years with atrophic acne scars, microneedling was performed on both halves of the face in a total of 3 treatment sessions spaced 1 month apart.7 Autologous PRP was given on the right half of the face whereas the other face was treated with distilled water. The Goodman’s qualitative scale showed excellent response in 40% of patients and good response in 60% of patients with the addition of PRP, whereas excellent response was noted in 10% and good response in 84% of patients, and poor response in 6% of patients. In a systematic review, the authors concluded that based on the paucity of data and small studies with few subjects, it is unclear whether addition of PRP to microneedling improves outcomes. Larger studies are required to determine its benefits in this setting.8
In radiofrequency (RF) microneedling, insulated (earlier generation devices) or non-insulated (more recent devices) deliver high-intensity RF energy into targeted tissue after the needle has broken through the skin. This results in collagenesis that leads to improvement in skin quality and texture. RF microneedling is a minimally invasive, nonablative methodology that can be used for body contouring, skin tightening, and cellulite reduction. RF microneedling has shown positive results in acne scarring, with improvements in skin texture, reduced pore size, decreased sebum production, and high patient satisfaction. At least 2 treatments are typically necessary for full results, but more may be needed. Compared to fully ablative procedures, RF microneedling results in minimal downtime. Further, radiofrequency is chromophore-independent, which allows higher penetration into the skin and is safe for the treatment of darker skin types. However, Fitzpatrick skin types IV-V may benefit from devices with shorter depths.
Lasers
Patients with either superficial or deep boxcar scars or rolling scars may be candidates for laser treatment. Both nonablative and ablative lasers have shown efficacy in improving acne scar appearance. Ablative lasers (typically CO2 or erbium YAG lasers) help to remove damaged scar tissue through melting, evaporation, or vaporization. In contrast, nonablative lasers (eg, NdYAG and diode lasers) do not remove tissue, but instead stimulate the formation of new collagen and result in skin tightening that causes the scar to raise to the skin surface.
The action of ablative lasers mainly occurs on the skin surface, but the depth of action is correlated to the intensity of the emitted energy and the diameter. Erbium lasers are almost exclusively ablative, whereas CO2 lasers ablate and also denature the tissues surrounding the ablation and result in increased myofibroblast and matrix protein (eg, hyaluronic acid) production. According to a systematic review, CO2 ablative fractional resurfacing achieved modest to excellent results in most studies, with the best responses in rolling and boxcar scars. However, side effects and complications are common. Scarring, infection, hyperpigmentation, and persistent erythema may be possible, and the risk of side effects must be considered when treating sensitive areas (eg, eyelids, upper neck, lower neck, and chest). Patients with a history of herpes virus skin infection within the 6 months prior to treatment, keloids, or hypertrophic scarring should be treated with caution by ablative laser. In addition, people with a high skin phototype (F 4-6) are at greater risk for hyperpigmentation than those with a low phototype (F 1-3). Hyperpigmentation may occur in 55% to 100% of patients, but the risk can be reduced by employing lower treatment densities and pulsed energies, avoiding sun exposure, and using pretreatment bleaching agents.
In a systematic review of 7 studies utilizing combination PRP fractional ablative laser treatment, 5 studies found added benefits to acne scar treatment, and symptom (eg, erythema, edema, and pain) improvement was noted in 6 of the 7 studies. However, variability in PRP preparation and delivery technique between studies complicates the analysis, and larger studies are needed to understand the parameters and determine the patient subtypes best suited for PRP with fractional ablative laser treatment.8
Nonablative lasers (fractional photothermolysis [FP]) cause thermal injury within the dermis but do not cause ablation in the epidermis. FP is most useful for boxcar and rolling scars, although results are generally moderate and the cosmetic results are generally inferior to ablative lasers. Nonablative therapies are associated with fewer side effects and shorter recovery time than treatment with ablative lasers, but may have higher levels of discomfort during the procedure.9
Fillers
Fillers are being used more frequently to treat facial acne scars, especially boxcar or rolling boxcar scars. Fillers are thought to improve the appearance of acne scars though augmentation of dermal and subcutaneous tissue scaffolding, in addition to the stimulation of collagen and tissue formation. For any application, it is important to consider the characteristics of the filler for the application, and the treatment of acne scars is no different. Variation in composition, biostimulatory capacity, flow properties, and duration of effect must be considered when choosing an appropriate filler for a given application. Goals when treating acne scars include skin-smoothing capacity and collagenesis stimulation and results that are durable over the long-term.
Fillers can be classified as stimulatory or replacement fillers (see Table 2). Hyaluronic acid (HA), is a replacement filler that is not indicated for treatment of acne scars, but it has shown improvement in acne scar appearance with minimal or transient side effects (eg, mild erythema) in 4 small studies of 2 to 12 patients each with follow-up ranging from 1 to 3 months10-13. Stimulatory fillers, such as poly-L-lactic acid (PLLA) and calcium hydroxylapatite (CaHA), have also shown positive results in the treatment of acne scars in smaller studies with a follow-up of up to 4 years with PLLA and 12 months with CaHA.14-19 Longer-lasting stimulatory fillers include polymethylmethacrylate (PMMA) and silicone, which have shown long-lasting results for the improvement of acne scars of up to 30 years for silicone and >5 years for PMMA.20,21
Table 2. Overview of Filler Types.
| Filler Type | Examples |
| Replacement | Hyaluronic acid (HA) |
| Stimulatory | Poly-L lactic acid (PLLA)Calcium hydroxyapatite (CaHA)PMMA- collagen gel |
PMMA collagen gel and CaHA are unique in that they can be placed more superficially than HA fillers. HA fillers, with the exception of Beletero Balance® (Merz Aesthetics, Sturtevant, WI) give rise to the Tyndall effect (also known as Rayleigh scattering), which refers to the bluish hue that is visible in the skin when HA is placed too superficially. This bluish tint can be distressing to patients and results in poor aesthetics outcomes, making HA a less desirable choice for acne scar treatment. PMMA collagen gel and CaHA are not subject to the Tyndall effect and may be placed more superficially than HA.
The only non-degrading, non-resorbable long-term filler is that FDA-approved and that has an indication to treat acne scars is Bellafill® (Suneva Medical, Inc., San Diego, CA), and the remainder of the chapter will focus on this agent. Bellafill® is a PMMA-collagen gel dermal filler that was originally manufactured by Artes Medical, Inc. and marketed under the brand name Artefill® from 2006 to 2008. Artes Medical, Inc. was purchased by Suneva Medical, Inc., and Artefill® was rebranded as Bellafill®. Notably, the PMMA-collagen gel dermal filler is identical in formulation and manufacturing process and has never been removed from the market. PMMA-collagen gel is FDA-approved for the correction of moderate to severe nasolabial folds and moderate to severe atrophic, distensible facial acne scars on the cheek in patients over the age of 21 years.22
PMMA is known to induce new dermal tissue matrix through a biostimulatory response. This response causes new dermal tissue to encapsulate the PMMA microspheres and may persist for up to 10 years or more.23 A histopathology study found that PMMA-collagen gel stimulates type III collagen within the first week of injection. Type III collagen peaks at month 2 and diminishes in months 3 through 6. Procollagen 1 was first noted after 1 month and increased in intensity and organization for up to 6 months. The PMMA microspheres remained localized to the initial injection area and were encapsulated with new collagen fibers, with the growth and pattern of new connective tissue mimicking those of a typical wound healing response.24
Filler rheological properties (ie, flow) are important clinical differentiators. The two most commonly measured rheological properties include complex viscosity and elastic modulus. Complex viscosity is a measurement of the resistance of a substance to nonrecoverable deformation (ie, loss of structural integrity) when shearing forces (ie, forces applied in opposite directions) are applied (eg, shearing forces during injection or forces from dynamic facial movement), and elastic modulus refers to the resistance of a substance to recoverable deformation when pressure is applied and is a quantitative measure of gel firmness or stiffness. Fillers with a higher elastic modulus require a smaller volume of product to generate equivalent lift and support for the overlying tissue, so an equal volume of filler is expected to have greater lifting capacity than a product with a lower elastic modulus. Of hyaluronic acid (HA), calcium hydroxylapatite (CaHA), and PMMA-collagen gel, PMMA-collagen gel exhibits the highest complex viscosity and elastic modulus. The high lifting capacity of Bellafill® contributes to its usefulness in treating acne scars, translating into a smaller amount of product needed to lift the overlying tissue. In addition, the product can be used in deeper layers of the skin where lift is needed.25 Further, Bellafill® is a long-lasting filler, and PMMA is not resorbed by the body. In studies of the mid-face, results have lasted >5 years.26 For applications such as acne scars in which long-term correction is desired, long-lasting duration is important.
Bellafill administration involves placement of the PMMA-collagen under the scar at the reticular dermal level or dermal subcutaneous junction, preferably using the micro-depot and retrograde linear threading technique. The technique typically will require the creation of a potential space within the dense fibrotic dermis directly underlying the scar, termed micro-subcision, which mimics traditional subcision. Depending on the scar, serial puncture may also be used. For linear threading, several passes are made in one direction, and several additional passes are made perpendicular to the original direction. A video detailing injection technique is shown (Video 1) Because Bellafill is a permanent filler, it is important to avoid overcorrection and instead rely on touch-up injections to achieve the best results. Bellafill may be used with or without laser augmentation. Bellafill contains 20% PMMA and 80% bovine collagen solution; the melting point of PMMA is 320°F, and thus there is no risk of the product melting at the energies used for acne scar treatment. Results achieved with PMMA-collagen gel are shown in Figures 1-3
Figure 1. 60-year old male treated with PMMA-collagen gel at baseline (A) and six months post injection (B). Treated scars are circled in yellow. The patient was treated with a total of 0.6cc PMMA-collagen gel (Images are courtesy of Suneva Medical, Inc)
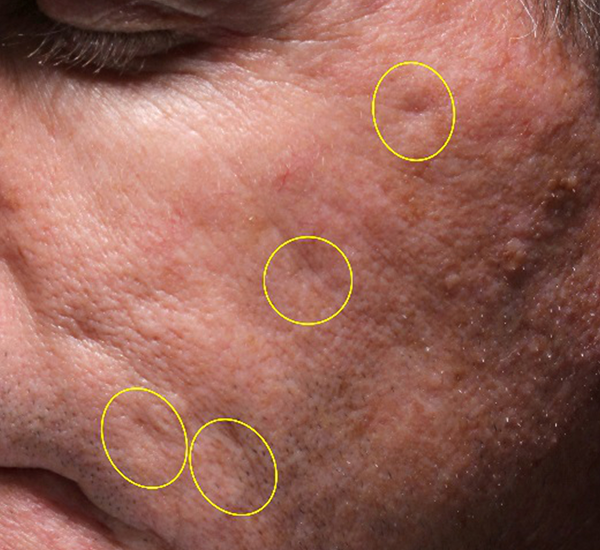
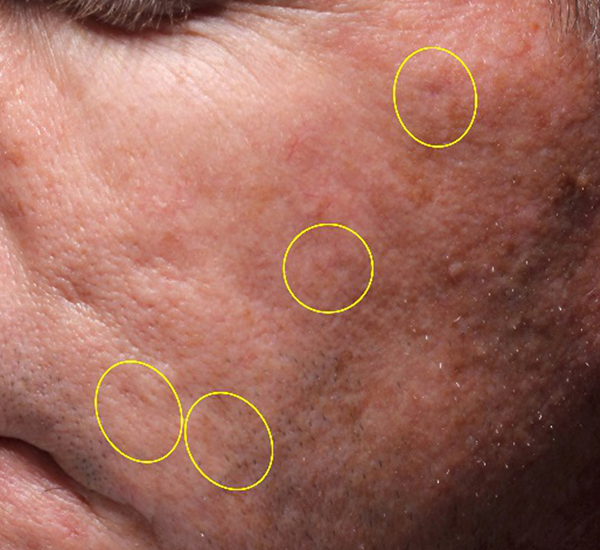
Figure 2. 56-year old female treated with PMMA-collagen gel at baseline (A) and at six months post injection (B). Treated scars are circled in yellow. The patient was treated with a total volume of 5.0cc of PMMA-collagen gel, 2.6cc at one treatment session and 1.8cc one month later. The change in global change appearance can be appreciated in C (Baseline) and D (six months) (Images are courtesy of Suneva Medical, Inc)
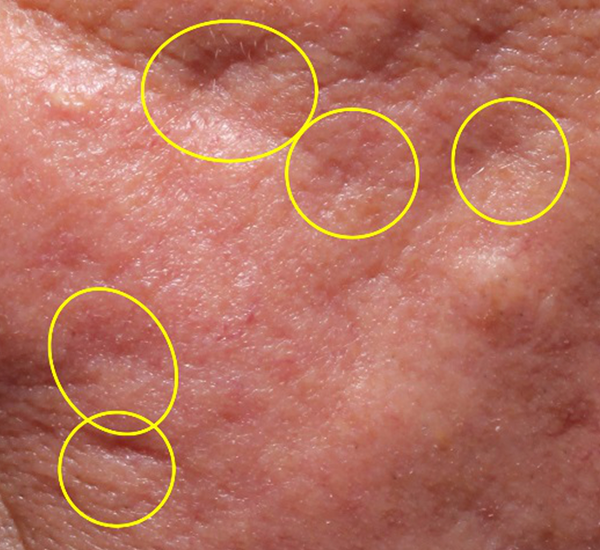
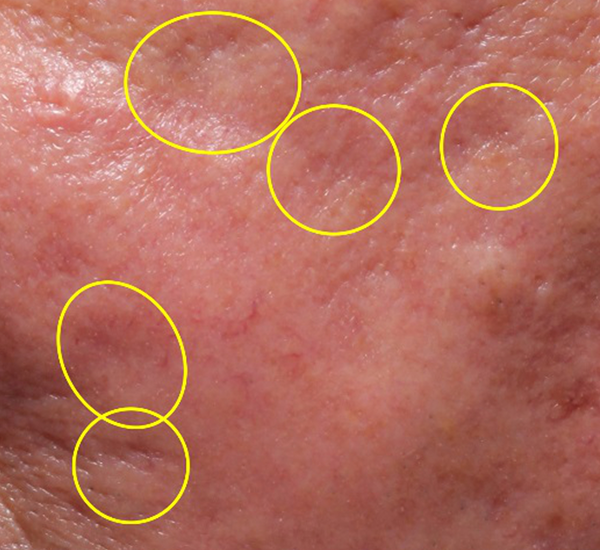
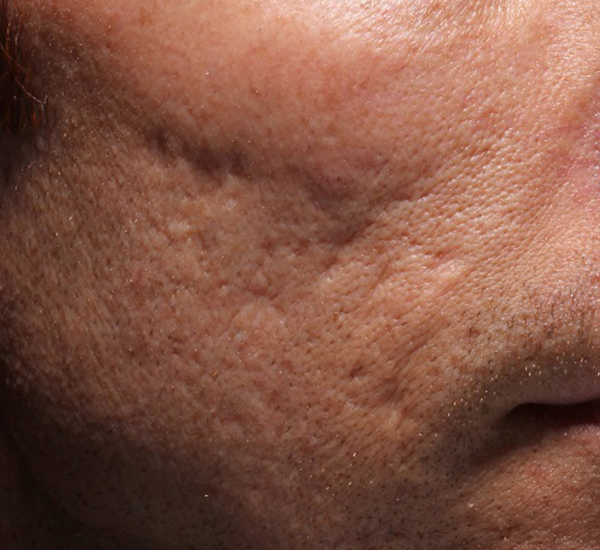
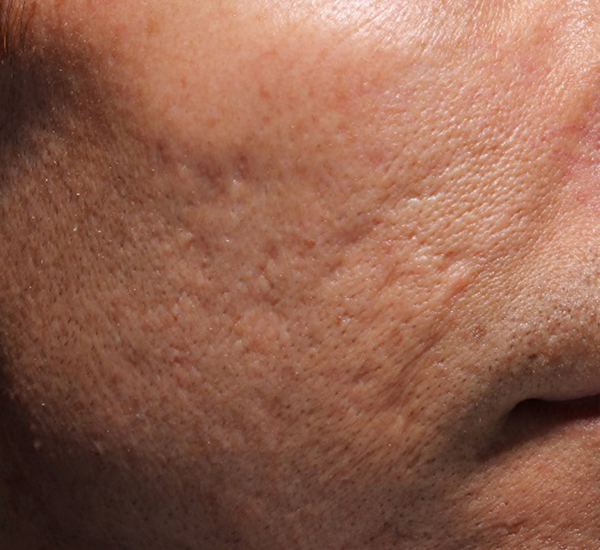
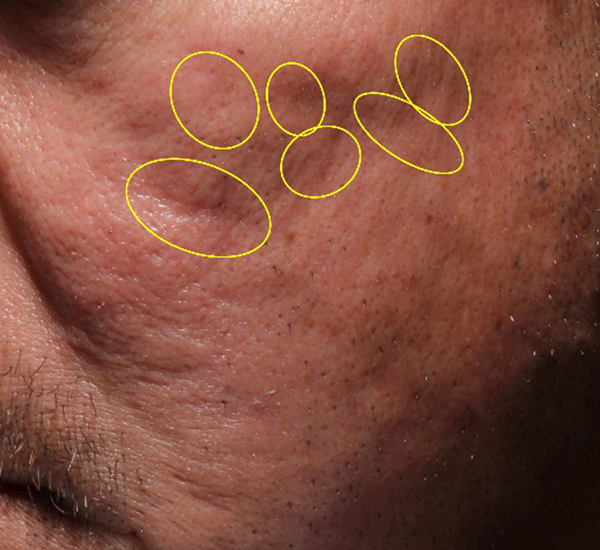
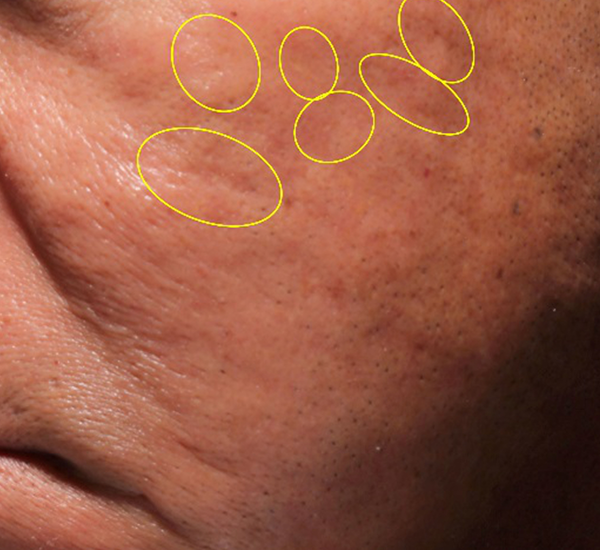
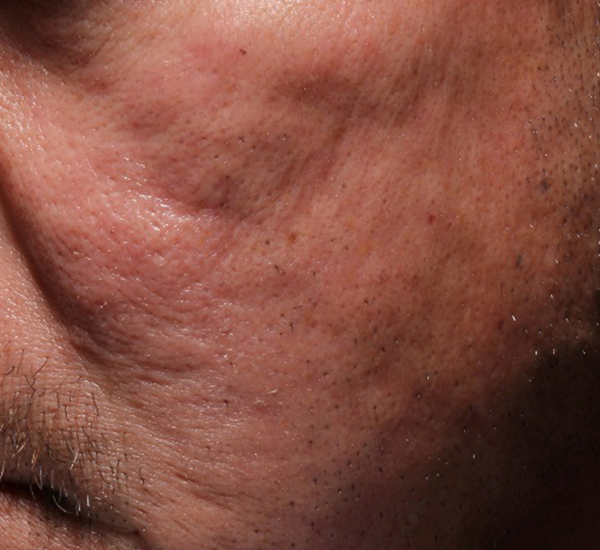
Figure 3. 49-year old male treated with PMMA-collagen gel at baseline (A) and at 11 months post injection (B). Treated scars are circled in yellow. The subject was treated with a total of 4.4 cc of PMMA-collagen gel, 2.6cc at the first visit, and 1.8cc at the second visit, one month later. The change in global change appearance can be appreciated in C (Baseline) and D (11 months). (Images are courtesy of Suneva Medical, Inc)
In a double-blind, randomized, multicenter, controlled trial of suspended PMMA microspheres for the correction of atrophic acne scars, 147 subjects received injections with PMMA-collagen gel (n=97) or saline (n=50). Scar severity was assessed using the Acne Scar Rating Scale (ASRS), where 1=minimal, 2=mild, 3=moderate, and 4=severe. After 6 months, significantly more subjects treated PMMA-collagen–treated subjects met the criteria for success, defined as a 2-point improvement in the ASRS score for at least 50% of scars, compared to control group (64% vs 33%, P=.0005). Impressively, 91% of PMMA-collagen–treated scars showed improvement, compared to 76% of those treated with saline. In addition, the Physician Global Aesthetic Improvement Scale demonstrated a significantly better response with PMMA-collagen than saline control (85% vs 54%, P=.0003). Overall, 84% of subjects treated with PMMA-collagen were satisfied or better compared to 52% of those treated with saline.21
However, common side effects with PMMA are generally mild and reversible and related to the micro-subcision that is an essential part of the technique. In more than 267 injections (177 with PMMA-collagen and 90 with saline), 21 adverse events (AEs) were reported in 17 of 97 (17%) subjects receiving PMMA-collagen, and 19 AEs were reported in 13 of 50 (26%) of subjects receiving saline injections. AEs that occurred in ≥2% of subjects that were determined to be related included injection-site pain, tenderness, and swelling. A total of 6 treatment-related AEs occurred in the PMMA-collagen group (2 reports each of injection site pain and bruising and 1 report each of injection-site pain and acne). Importantly, there were no granuloma occurrences were reported.21
Because PMMA is a permanent, non-resorbable filler, use of proper injection technique is of the utmost importance to ensure the highest level of efficacy and safety due to the potentially irreversible nature of adverse events.27 Granulomas result from a tissue reaction to filler and consist of an inflammatory infiltrate, including histiocytes and epithelioid cells. Clinically, it can develop slowly or quickly, though the typical time between injection and granuloma appearance is 6 to 24 months. Notably, granuloma formation occurs less frequently with fillers with smooth surfaces (eg, Bellafill®) than those with irregular surfaces. Untreated granulomas can last for years and may remain unchanged in clinical appearance, increase in size, or resolve spontaneously. Generally, granulomas respond well to intralesional or systemic corticosteroids. It is important to note that with proper injection technique, granuloma formation is extremely low, and none were reported in the pivotal acne studies.
Hranuloma formation is estimated to occur with PMMA microspheres at a frequency of around 0.02%.28 A recent report on post-market surveillance data of PMMA-collagen gel reported a total of 66 granulomas in 839 patients with an overall complaint rate of 0.009% for histology-confirmed granuloma and a 0.009% complaint rate for histology-unconfirmed granulomas, across all treatment areas, including off-label use (submitted manuscript).
Conclusion
Various treatments are available to drastically reduce the appearance of moderate to severe acne scars in affected individuals, including microneedling with or without platelet-rich plasma, laser (alone or in conjunction with other treatments), and fillers. In particular, Bellafill® is the only permanent filler that is FDA-approved for use in treating moderate to severe atrophic acne scars. As always, appropriate injection technique is important to ensure the best treatment results and reduce the incidence of side effects.
Acknowledgements
The authors would like to thank Ginny Vachon, PhD and Cecelia Wall, MS of Principal Medvantage, LLC, Atlanta, GA for assistance in writing this manuscript. Funds for editorial support were provided by Suneva Medical, LLC.
References
- Dreno B, Khammari A, Orain N, et al. ECCA grading scale: an original validated acne scar grading scale for clinical practice in dermatology. Dermatology. 2007;214(1):46-51.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32(12):1458-1466.
- Goodman GJ, Baron JA. Postacne scarring–a quantitative global scarring grading system. J Cosmet Dermatol. 2006;5(1):48-52.
- El-Domyati M, Barakat M, Awad S, Medhat W, El-Fakahany H, Farag H. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8(7):36-42.
- Minh PPT, Bich DD, Hai VNT, et al. Microneedling therapy for atrophic acne scar: effectiveness and safety in Vietnamese patients. Open Access Maced J Med Sci. 2019;7(2):293-297.
- Fernandes D, Signorini M. Combating photoaging with percutaneous collagen induction. Clin Dermatol. 2008;26(2):192-199.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15(4):434-443.
- Hesseler MJ, Shyam N. Platelet-rich plasma and its utility in the treatment of acne scars: a systematic review. J Am Acad Dermatol. 2019;80(6):1730-1745.
- Rongsaard N, Rummaneethorn P. Comparison of a fractional bipolar radiofrequency device and a fractional erbium-doped glass 1,550-nm device for the treatment of atrophic acne scars: a randomized split-face clinical study. Dermatol Surg. 2014;40(1):14-21.
- Halachmi S, Ben Amitai D, Lapidoth M. Treatment of acne scars with hyaluronic acid: an improved approach. J Drugs Dermatol. 2013;12(7):e121-123.
- Goodman GJ, Van Den Broek A. The modified tower vertical filler technique for the treatment of post-acne scarring. Australas J Dermatol. 2016;57(1):19-23.
- Patel T, Tevet O. Effective treatment of acne scars using pneumatic injection of hyaluronic acid. J Drugs Dermatol. 2015;14(1):74-76.
- Hasson A, Romero WA. Treatment of facial atrophic scars with Esthelis, a hyaluronic acid filler with polydense cohesive matrix (CPM). J Drugs Dermatol. 2010;9(12):1507-1509.
- Sapra S, Stewart JA, Mraud K, Schupp R. A Canadian study of the use of poly-L-lactic acid dermal implant for the treatment of hill and valley acne scarring. Dermatol Surg. 2015;41(5):587-594.
- Sadick NS, Palmisano L. Case study involving use of injectable poly-L-lactic acid (PLLA) for acne scars. J Dermatolog Treat. 2009;20(5):302-307.
- Sadove R. Injectable poly-L: -lactic acid: a novel sculpting agent for the treatment of dermal fat atrophy after severe acne. Aesthetic Plast Surg. 2009;33(1):113-116.
- Rkein A, Ozog D, Waibel JS. Treatment of atrophic scars with fractionated CO2 laser facilitating delivery of topically applied poly-L-lactic acid. Dermatol Surg. 2014;40(6):624-631.
- Beer K. A single-center, open-label study on the use of injectable poly-L-lactic acid for the treatment of moderate to severe scarring from acne or varicella. Dermatol Surg. 2007;33 Suppl 2:S159-167.
- Goldberg DJ, Amin S, Hussain M. Acne scar correction using calcium hydroxylapatite in a carrier-based gel. J Cosmet Laser Ther. 2006;8(3):134-136.
- Carvalho Costa IM, Salaro CP, Costa MC. Polymethylmethacrylate facial implant: a successful personal experience in Brazil for more than 9 years. Dermatol Surg. 2009;35(8):1221-1227.
- Karnik J, Baumann L, Bruce S, et al. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014;71(1):77-83.
- Bellafill [instructions for use]. San Diego, CA: Suneva Medical, Inc.; July 2020.
- Lemperle G, Knapp TR, Sadick NS, Lemperle SM. ArteFill permanent injectable for soft tissue augmentation: I. Mechanism of action and injection techniques. Aesthetic Plast Surg. 2010;34(3):264-272.
- Ronan SJ, Eaton L, Lehman A, Pilcher B, Erickson CP. Histologic characterization of polymethylmethacrylate dermal filler biostimulatory properties in human skin. Dermatol Surg. 2019;45(12):1580-1584.
- Forbat E, Ali FR, Al-Niaimi F. The role of fillers in the management of acne scars. Clin Exp Dermatol. 2017;42(4):374-380.
- Cohen S, Dover J, Monheit G, et al. Five-year safety and satisfaction study of PMMA-collagen in the correction of nasolabial folds. Dermatol Surg. 2015;41 Suppl 1:S302-313.
- Fitzgerald R, Bass LM, Goldberg DJ, Graivier MH, Lorenc ZP. Physiochemical characteristics of poly-L-lactic acid (PLLA). Aesthet Surg J. 2018;38(suppl_1):S13-S17.
- Lemperle G, Gauthier-Hazan N, Wolters M, Eisemann-Klein M, Zimmermann U, Duffy DM. Foreign body granulomas after all injectable dermal fillers: I. possible causes. Plast Reconstr Surg. 2008;122:1-22.
