The authors declare no conflicts of interest with respect to the authorship and/or publication of this article.
The authors received no financial support for the research and/or authorship of this article.
Abstract
Patients are increasingly looking for alternative, non-surgical options to address submental fullness. ATX-101 (Kybella) is a synthetic form of deoxycholic acid that works by selectively targeting adipocytes for cell lysis, and was FDA approved to target submental fat in 2015. Patients with unwanted submental fullness primarily as a result of the accumulation of pre-platysmal fat are ideal candidates. This chapter will provide an overview of the mechanism of action, FDA approval process, patient selection, injection technique, and expanded indications.
Introduction
The contour of the chin and neck play a prominent role in the ideal facial aesthetic. A strong chin is considered desirable and projects self-confidence and authority. In contrast, submental convexity, perceived as a “double chin,” can make an individual appear older or overweight.1 The increasing popularity of social media and video conferencing, as well as the “selfie” culture, has also placed an emphasis on the appearance of this area. Survey results have demonstrated that this is a problem area for many, with over 70% of respondents reporting that they are bothered by the appearance of excess fat under the chin.2 The accumulation of submental fat (SMF) can contribute to submental fullness. However, this area often cannot be fully addressed with weight loss alone, as age and genetics often play a disproportionate role in the accumulation of SMF.1,2
Submental fat is distributed between the pre-platysmal and post-platysmal compartments. Cadaver dissections suggest that on average, 70% of SMF is pre-platysmal, although this distribution can vary significantly among individuals.1 Pre-platysmal fat is usually targeted because removal of post-platysmal fat can cause a concave and distorted neck appearance. Patients are increasingly looking to target SMF with both surgical and non-surgical options. Traditionally surgical options include liposuction, combined face/neck lift, and direct fat excision.2 Chin augmentation has also become increasingly popular. However, as cosmetic medicine has shifted to become more minimally invasive, there has been increased demand for non-surgical treatments to address SMF. These trends led to the popularity of Lipodissolve, a lipolytic injection containing a combination of phosphatidylcholine (PC) and deoxycholic acid (DCA), which for many years was used off-label to reduce unwanted deposits of localized fat.2 However, a lack of clinical trial data as well as reports of severe side effects including multisystem organ failure led the FDA to issue warnings against use of this product in 2010.
Despite concerns about the safety of Lipodissolve, its widespread off-label use prompted scientists to characterize its mechanism of action in vitro, where it was shown to target adipocytes and cause cell lysis. Ultimately, DCA was identified as the active adipocytolytic ingredient in the compound and was found to cause lipolysis by targeting fat cells specifically without injury to other tissues. This observation prompted the development of a synthetic form of deoxycholic acid, ATX-101 (Kybella) by Kythera Biopharmaceuticals, Inc in 2016.3 Starting in 2007, ATX-101 became the focus of an extensive pre-clinical research effort that included in vitro and later in vivo studies to further characterize the mechanism of action of ATX-101.1
Kybella Mechanism of Action
Deoxycholic acid is an endogenous secondary bile acid that solubilizes dietary fat in the gut.1 The synthetic form, ATX-101, is identical in structure but without human- or animal-derived contaminants. DCA acid acts as a detergent to disrupt the cell membrane, resulting in cell death. In vitro models show that DCA causes adipocyte death preferentially, resulting in a reduction in the overall number of adipocytes, and that the presence of albumin or other proteins decreases the activity of DCA so that non-fat tissues such as skin and muscle remain unaffected.1 It is hypothesized that adipocytes are preferentially targeted in vivo due to the absence of protein binding and subsequent inactivation.3 Microscopic evaluation of tissue treated with DCA shows that the compound causes adipocytolysis as early as day 1, local inflammation with infiltration of neutrophils and T cells by day 3, macrophage invasion by day 7, and tissue remodeling by fibroblasts with collagen deposition by day 28, at which time inflammation has largely resolved.4
FDA Approval Process
Following these pre-clinical studies, phase I trials were launched to evaluate the safety and pharmacokinetics of ATX-101.3 These studies demonstrated that after injection into subcutaneous tissues, plasma concentrations of DCA peaked rapidly and returned to baseline within 24 hours. Administration of DCA had no effect on plasma levels of total cholesterol, triglycerides, free fatty acids or C-reactive protein, and no clinically meaningful impact on liver or kidney function.2 Adverse effects appeared mild and temporary, and included injection-site pain, erythema, and hematoma.
Phase II trials established the optimal dosing and treatment regimen. Two multicenter, randomized, double-blind, placebo-controlled studies demonstrated that 2 mg/cm2 dose resulted in greater efficacy compared to 1 mg/cm2, but there was no added benefit with a dose of 4 mg/cm2, which was associated with an increased rate of adverse events. The 2 mg/cm2 dose was achieved via 0.2 mL injections spaced at 1 cm intervals, with 28 days in between treatment sessions. This is now the approved treatment protocol for ATX-101.3
The ATX-101 phase III clinical trials included several large trials in Europe, as well as two multicenter, double-blind, placebo-controlled pivotal trials in the U.S. and Canada, referred to as REFINE-1 and REFINE-2, respectively, to evaluate the efficacy and safety of the compound.1 The REFINE trials enrolled a combined total of 1,022 patients and evaluated a single dose strength (2 mg/cm2) for up to 6 treatments, with the end point being SMF reduction at 12 weeks following the last treatment. Primary end points included the proportion of subjects who achieved a ≥ 1 grade improvement on both the Clinician-Reported Submental Fat Rating Scale (CR-SMFRS) and the Patient-Reported Submental Fat Rating Scale (PR-SMFRS), and the proportion of patients who achieved a ≥ 2 grade improvement on both scales. Secondary end points included the proportion of subjects who achieved ≥ 10% reduction in SMF volume on magnetic resonance imaging (MRI), and the psychological effect of treatment based on the Patient-Reported Submental Fat Impact Scale (PR-SMFIS).1
In REFINE-1, more patients in the treatment group achieved a ≥ 1-point improvement in CR-SMFRS/PR-SMFRS compared to those treated with placebo (70% vs 18.6%, p < 0.001), and 13.4% in the treatment group achieved a ≥ 2-point improvement in CR-SMFRS/PR-SMFRS compared to 0% in the placebo group (p < 0.001).3 For the pooled analysis of REFINE-1 and REFINE-2, results were similar to results from REFINE-1 and the large European trials, with significantly more patients in the treatment group achieving ≥ 1-point improvement on the CR-SMFRS (68.2%) compared to placebo (20.5%, p < 0.001).4 In REFINE-1, significantly more patients treated with ATX-101 were considered MRI responders compared to placebo (46.3% versus 5.3%, p < 0.001). Fifty-five percent of patients responded within 2 treatment sessions and 75% within 4 treatment sessions.4
Across phase III trials, the majority of adverse events (AEs) were related to the injection site and included pain, swelling, hematoma (bruising), numbness, erythema, induration, and nodule formation. Injection site AEs were reported in ≥ 10% of patients in the treatment groups.1 Most AEs were mild and resolved within 28 days, and were most commonly associated with the initial session. Marginal mandibular nerve (MMN) paresis was reported in 4% of patients in the REFINE trials, comparable to rates of MMN paresis reported with liposuction or cervical rhytidectomy.3 All instances resolved completely, ranging from 7 to 61 days.
Although the recommendation is to space treatments at 28-day intervals, post hoc analysis of data from a phase IIIb study demonstrates that reduction of SMF continues for at least 2-3 months after a single treatment.5 These findings suggest that efficacy may build over time, with the initial inflammatory response resolving by day 28 and subsequent tissue remodeling and neocollagenesis occurring over subsequent months.
Patient Selection and Counseling
Proper patient selection is of the utmost importance and is perhaps the strongest predictor of treatment success. Unlike other treatments of the submental area (facelift, neck lift, liposuction) only the subcutaneous, pre-platysmal layer of SMF is treated. Patients with significant skin laxity or platysmal banding are not ideal candidates. Additionally, DCA treatment may unmask platysma bands previously hidden beneath pre-platysmal fat. It is important to get a full medical and surgical history in order to identify any history of prior treatments or surgeries that may distort facial anatomy.2 Patients should be counseled that best results may be achieved by undergoing a series of treatments spaced 28 days apart. The number of treatments is determined by the thickness of the submental fat, and it is common for a patient to require between 2 and 6 total treatments to achieve the desired result. Patients should also be counseled about common immediate adverse events, including swelling, inflammation, and pain at the injection site. Proper analgesia should be offered, and patients should be instructed to avoid the use of anticoagulants or antiplatelets 7-10 days before injection.6
Injection Technique
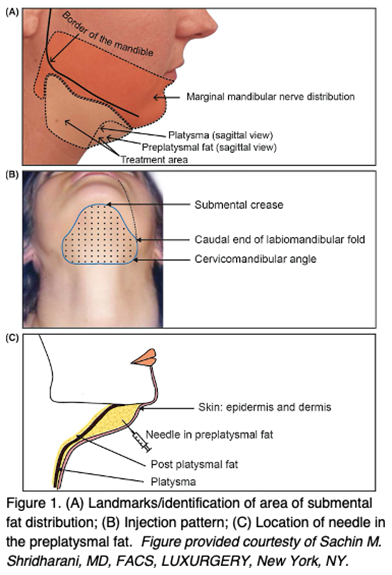
As discussed previously, DCA initiates adipocyte destruction immediately upon injection, which results in localized swelling, inflammation, and pain. While analgesia is not absolutely necessary, it is highly recommended as many simple methods exist that greatly increase the comfort of the procedure. Major recommendations include pretreatment with ibuprofen and/or acetaminophen one hour prior to treatment, as well as an injection of lidocaine with epinephrine 15 minutes before DCA injection.6 Cold packs can also be administered before or after the procedure.
Prior to marking the patient, the skin is thoroughly cleansed and dried. Next, it is important to define the patient’s anatomy and the intended injection zone. A skin marker is used to mark the superior extent of the injection area at the submental crease. The inferior extent is marked at the level of the hyoid, and the lateral extent is marked by dropping a line from the oral commissures bilaterally and intersecting the two horizontal lines. This rectangle defines the injection treatment zone.2 It is important to remain 1 to 1.5 cm below the lower border of the mandible in order to avoid injury to the marginal mandibular nerve; thus, the treatment zone may need to be modified if the area does overlap the marginal mandibular nerve “danger zone.”
Kybella is packaged in four sterile, 2 mL vials of 10 mg/mL concentration. The FDA approved dose is 0.2 mL spaced at 1-cm intervals (2 mg/cm2). An injection grid is provided with colored dots 1 cm apart for accurate spacing. This grid is applied to the pre-designated injection zone, and additional dots outside the zone are removed or ignored. Each vial of DCA allows for 10 individual injections. In order to easily determine the amount needed, one count the total number of dots within the injection zone and divide by 10 to quickly calculate the total number of vials required for treatment. Any necessary anesthesia is then applied prior to beginning the injections. The solution is drawn up into 1 mL tuberculin syringes and the injections are performed with a 30 gauge or smaller needle. The needle is inserted into the skin, and 0.2 mL is injected at each dot. To ensure the injection is not too superficial, it is advisable to pinch the skin with two fingers to identify the subcutaneous fat later and ensure the needle traverses to an appropriate depth.2 One treatment session can encompass up to 50 injections total. Due to the expected swelling and bruising after the procedure, patients are advised to allocate 2 to 3 days for recovery.
Clinical Experience with ATX-101
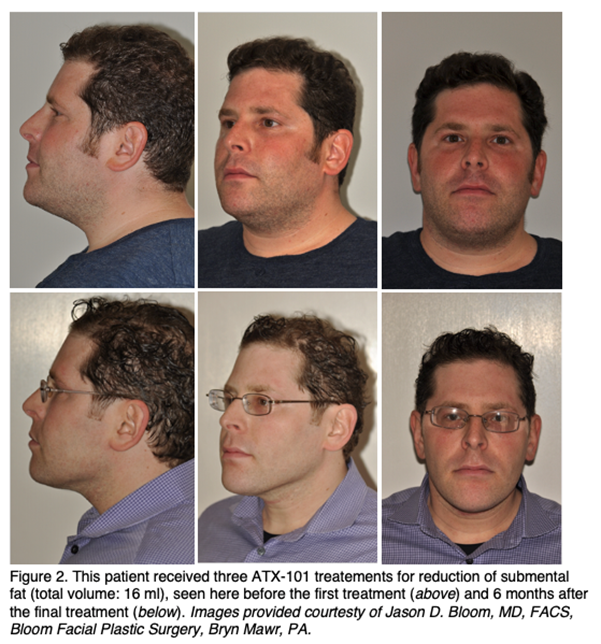
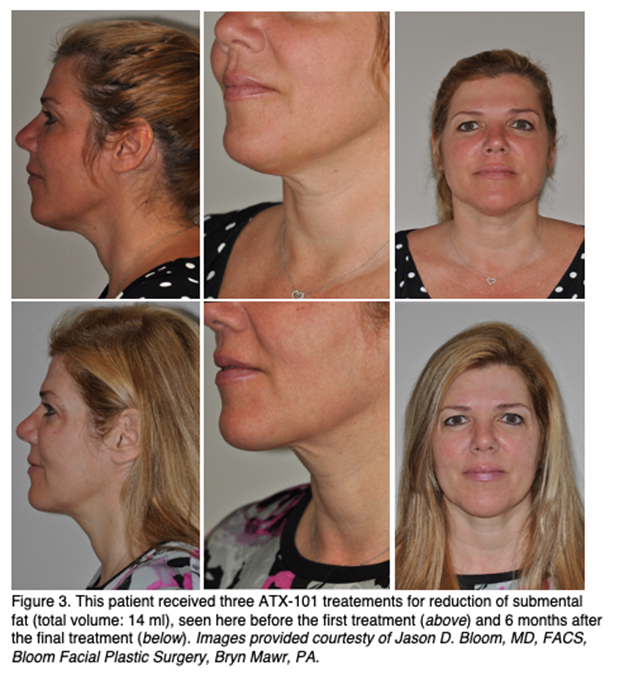
In 2017, Shridharani reported results from the first series of 100 patients treated with ATX-101 in private practice or an academic setting since the product became commercially available.7 He reported that compared to the phase III trial data, patients in this series on average underwent fewer sessions and had less total volume injected. Of the 100 patients enrolled in the series, 58 underwent only one treatment. Of these, 79% were considered responders, defined as a ≥ 1 point improvement in CR-SMFRS as determined by the study author and two blinded physicians. Of the 42 patients who underwent multiple treatments, all responded by 1-2 points. A two-year follow-up study by Shridharani notes that of the 58 who had undergone only one treatment in the initial analysis, 17 returned for additional sessions, bringing the multiple treatment group to 59.8 All of these patients responded by ≥ 1 point on the CR-SMFRS.
Shridharani notes that compared to the phase III trials, patients in his clinical practice tend to undergo fewer sessions and have less total volume injected. This may be attributed to the financial burden of multiple sessions outside of a clinical trial setting. He notes that patients are likely to require > 1 session to achieve their desired result, but an improvement in CR-SMFRS score is often seen after just one treatment. Interestingly, he observes that men tend to require a larger injection volume due to a larger surface area, and are more likely to return for multiple sessions.8,9 Another prospective series of 202 patients by Humphrey et al similarly notes that treatment continuity remains a challenge in clinical practice.10 Compared to a median of 4.7 treatments in phase III trials, patients in a clinical setting complete a median of just 1.7 treatments. It is therefore recommended to emphasize the need for at least 2 sessions up front, and to streamline the booking of multiple appointments to ensure continuity.
Adverse Events
The rate of adverse events reported in clinical practice is similar to what is reported in the phase III data. The most commonly reported AEs are related to the injection site and include pain, swelling, bruising, paresthesias, and nodules. Transient alopecia around the injection site has also been reported in a male patient.8
Perhaps the most feared adverse event is paresis of the marginal mandibular nerve (MMN), which has been reported in 2-4% of patients receiving ATX-101 injection, all of which were transient.3,7 It is hypothesized that this transient injury is related to damage to the myelin sheath surrounding the nerve leading to temporary demyelination and inflammation, rather than to direct intra-neural injury.9 In order to avoid trauma to the nerve, it is recommended that ATX-101 not be injected superior to the inferior border of the mandible, or within a region defined by a 1.0-1.5 cm line below the inferior border of the mandible. Although vascular injury was not reported in published phase III data, there have been subsequent reports of vascular injury after ATX-101 injection. These episodes were characterized by pain, blanching, and dusky discoloration in the distribution of the submental artery immediately after injection.11 All instances were successfully treated with warm compresses, massage, topical nitroglycerine, prednisone and aspirin, with no permanent sequelae.
Expanded Indications
ATX-101 is FDA approved for use in the submental area, but additional studies have reported successful use for expanded indications. The traditional submental “safe zone” is defined by the hyoid inferiorly, the submental crease superiorly, and the lateral extent of the bilateral oral commissures. In 2019 Shridharani reported a retrospective review describing an expanded “safe zone” to isolate distinct fat compartments that remain untreated by the traditional safe zone while still avoiding the MMN “danger zone.”12 This expanded zone is subdivided into S1 (the original safe zone), S2 (extending from the oral commissure to the antegonial notch), S3 (extending laterally still to the anterior border of the sternocleidomastoid muscle), and S4 (from the thyroid notch border extending inferiorly to the inferior neck crease and bound laterally by the SCM). A no-treatment zone is defined by the area 2.0 cm inferior to the mandible at the antegonial notch. Shridharani notes that not all zones need to be treated in every patient, but that specific areas can be targeted at the discretion of the clinician. He notes a 95.8% response rate with this technique, with the lateral fat zones often improving first and subsequent treatments required for S1. The rate of temporary MMN paresis (4.8%) was noted to be comparable with previous studies.
In 2020, Shridharani reported additional expanded use of ATX-101 for the treatment of the jowl fat pads.13 Injections were performed in the area superior to the inferior mandibular border, anterior to the antegonial notch and posterior to the caudal continuation of the oral commissure. Of note, patients with jowling secondary to extreme skin laxity were excluded. Improvement was judged based on standardized photographs at baseline and final follow-up, and improvement was noted in 98% of patients. Because the terminal MMN arbolizes deep to the subcutaneous fat at the jowl, the study author recommends pinching and retracting the skin and subcutaneous fat at the injection site to minimize the likelihood of paresis.
Conclusion
ATX-101 (Kybella) is a synthetic form of deoxycholic acid and works by selectively targeting adipocytes for cell lysis by disrupting the cellular membrane. ATX-101 was FDA approved in 2015 after a series of clinical trials demonstrated its safety and efficacy. Patient selection remains of the utmost importance for achieving optimal treatment results, with the ideal patient demonstrating submental fullness secondary to submental fat rather than excess skin laxity, submandibular gland ptosis, or platysmal banding. Patients should be counseled that best results are achieved with ≥ 2 sessions. Adverse events tend to be mild and temporary, although temporary marginal mandibular nerve paresis has been reported in 2-4% of patients. Successful use of Kybella for expanded indications in the submental area and jowl area has been reported.
- Ascher B, Fellmann J, Monheit G. ATX-101 (Deoxycholic Acid Injection) for Reduction of Submental Fat. Expert Rev Clin Pharmacol. 2016 Sep;9(9):1131-1143.
- Liu M, Chesnut C, Lask. Overview of Kybella (Deoxycholic Acid Injection) as a Fat Resorption Product for Submental Fat. Facial Plast Surg. 2019 Jun;35(3):274-277.
- Georgesen C, Lipner SR. The Development, Evidence, and Current Use of ATX-101 for the Treatment of Submental Fat. J Cosmet Dermatol. 2017 Jun; 16(2): 174-179.
- Dunican KC, Patel DK. Deoxycholic Acid (ATX-101) for Reduction of Submental Fat. Ann Pharmacother. 2016 Oct;50(10):855-861.
- Dover JS, Shridharani SM, Bloom JD, et al. Reduction of Submental Fat Continues Beyond 28 Days After ATX-101 Treatment: Results From a Post Hoc Analysis. Dermatol Surg. 2018 Nov;44(11):1477-1479.
- Fagien S, McChesney P, Subramanian M, et al. Prevention and Management of Injection-Related Adverse Effects in Facial Aesthetics: Considerations for ATX-101 (Deoxycholic Acid Injection) Treatment. Dermatol Surg. 2016 Nov;42 Suppl 1:S300-304.
- Shridharani S. Early Experience in 100 Consecutive Patients With Injection Adipocytolysis for Neck Contouring With ATX-101 (Deoxycholic Acid). Dermatol Surg. 2017 Jul;43(7):950-958.
- Shridharani S. Real-World Experience With 100 Consecutive Patients Undergoing Neck Contouring With ATX-101 (Deoxycholic Acid): An Updated Report With A 2-Year Analysis. Dermatol Surg. 2019 Oct;45(10):1285-1293.
- Shridharani S, Behr KL. ATX-101 (Deoxycholic Acid Injection) Treatment in Men: Insights From Our Clinical Experience. Dermatol Surg. 2017 Nov;43 Suppl 2:S225-S230.
- Humphrey S, Femmer P, Beleznay K, et al. Deoxycholic Acid for Submental Fullness and More: Real-World Experience With 202 Patients. Dermatol Surg. 2019 Apr;45(4):624-627.
- McKay C, Price C, Pruett L. Vascular Injury After Deoxycholic Acid Injection. Dermatol Surg. 2019 Feb;45(2):306-309.
- Shridharani SM, Chandawarkar AA. Novel Expanded Safe Zone for Reduction of Submental Fullness With ATX-101 Injection. Plast Reconstr Surg. 2019 Dec;144(6):995e-1001e.
- Shridharani SM. Improvement in Jowl Fat Following ATX-101 Treatment: Results From a Single-Site Study. Plast Reconstr Surg. 2020 Apr;145(4)929-935.
