Correspondence to: Dr. Sachin M. Shridharani, LUXURGERY, 880 Fifth Avenue #1B/C/D, New York, NY 10021, USA. E-mail: sms@luxurgery.com
Introduction
Patient demand for nonsurgical alternatives to aesthetic surgery has increased dramatically in recent years. In 2019, more than 16.3 million nonsurgical cosmetic procedures were performed in the United States alone, demonstrating a 237% increase since the year 2000.1 Among the available nonsurgical cosmetic treatments, body contouring procedures in particular have demonstrated large and consistent growth. In 2015, injectable deoxycholic acid (DCA; Kybella®, Allergan Plc [Dublin, Ireland]) became the first FDA-approved pharmacological agent for injection adipocytolysis and is indicated for the reduction of excess submental fat.2
Deoxycholic acid is a secondary bile acid produced endogenously in humans that functions to emulsify and solubilize dietary fats during digestion.3 Injectable DCA is a preparation of synthetic deoxycholic acid 10 mg/ml for subcutaneous use.2 When injected subcutaneously, DCA irreversibly disrupts the cell membranes of adipocytes causing cell lysis (adipocytolysis) and a subsequent reduction in fat volume.4-6 This stimulates a mild inflammatory response followed by macrophage-induced phagocytosis of the adipocyte remnants. The inflammatory response associated with DCA injection initiates physical and histological tissue changes (e.g. fibrosis and dermal thickening) which have been observed to result in moderate skin tightening.7 Following subcutaneous administration, exogenous DCA joins the endogenous bile acid pool in the enterohepatic circulation. Subsequently, exogenous DCA is excreted intact along with the endogenous bile acids.2
While injectable DCA is currently FDA approved for the reduction of submental fullness, non-submental applications of DCA injection are common in clinical practice. The injectable nature of DCA and its impressive lipolytic properties make it an ideal agent for targeting focal fat deposits, particularly in small areas of the body that are not conducive to treatment with large cannulas or device applicators. The authors were among the earliest to implement DCA injection for non-submental body contouring and have substantial clinical experience using injectable DCA outside of the submental region. This chapter will discuss important considerations relating to the use of DCA for non-submental body contouring.
General Principles
Patient Selection
While injection adipocytolysis can be effective in many patients, it is critical for practitioners to understand when ATX-101 injection is appropriate for addressing a patient’s concerns and when it is not. This requires judicious patient selection and close attention to the nature of the area being treated. Of note, the only absolute contraindication to ATX-101 injection is active infection within the treatment area.
Full, Not Floppy. Though clinical observation of modest skin tightening has been reported, ATX-101 functions primarily as a lipolytic agent with safe utilization demonstrated in the subcutaneous layer. Suitable patients should have excess subcutaneous fat in the area of concern without significant overlying skin laxity. Physical exam by pinch or palpation should elucidate whether the cause of deformity is attributable primarily to subcutaneous fat, or to deeper fat compartments/structures; deeper fat compartments cannot be treated safely with ATX-101. Men in particular may present with visceral or deep fat not treatable by ATX-101 and should be counseled appropriately.
Elucidate Etiology. In all cases, it is prudent to elucidate the etiology of the observed deformity and screen for potential causes other than excess fat. This is to confirm that the observed deformity is purely subcutaneous lipodystrophy and can be safely treated with ATX-101 without the need for further workup. These considerations are specific to the region under assessment and will be touched upon in the following sections.
Pre-Procedure Planning & Preparation
To identify the target treatment region, patients should undergo physical and local examination of the intended treatment area. Prior to administering treatment, practitioners should assess vital signs, obtain a detailed medical history, and evaluate for bleeding disorders. It is important to counsel the patient that following treatment he/she will experience a period of edema secondary to the inflammatory response induced by ATX-101. The patient can be reassured that this is an anticipated part of the recovery process that signals the lipolytic process. While post-treatment swelling can be pronounced, it generally resolves by day 5 to 10 depending upon the degree of treatment and the amount of medication injected.
As with any aesthetic procedure, pre-treatment photography should be undertaken to document response. Patients should be counseled on the probability of needing 2-4 treatments at 6 weeks intervals in order to achieve optimal results.
Technique
ATX-101 injection is generally performed as a semi-sterile procedure. The treatment area can be prepped with standard alcohol wipes or chlorhexidine prior to marking.
Positioning & Markings
Positioning and markings will vary based on the treatment region. It is important to remember that positioning may distort deformities, especially in dependent areas. For this reason, the authors recommend marking the patient in an upright position to ensure accurate representation. Any important anatomical landmarks or structures should also be marked. Because the lipolytic effect of ATX-101 can extend beyond the injection boundary and demyelinate adjacent nerves, neurovascular structures must be avoided using a >2 cm buffer zone.
Once initial markings are in place, it may be prudent to review the location and nature of the injections with the patient to best address the patient’s needs and to ensure optimal contouring. After the markings are confirmed, the patient should be placed in a comfortable position (usually reclined). This allows ease of access for the injector as well as enhanced patient comfort during a sometimes-uncomfortable procedure.
The 1-cm injection grid should be applied to the treatment area to mark the injection sites. The grid can be trimmed to fit within small treatment areas or multiple grids can be used to cover large treatment regions. Use of the injection grid – or a comparable alternative – is imperative to ensure injection sites are equally spaced by 1 cm. The diffusion zone of ATX-101 in the subcutaneous space is 1 cm2. Injection adherence to the grid ensures an equal distribution and diffusion of the medication, leading to a uniform outcome. The grid can be applied using a gauze soaked with sterile water or saline. Any grid points that fall outside of the marked treatment zone should be removed with alcohol wipes.
Local Anesthesia
Treating small areas of excess fat may not require the use of local anesthesia, however, local anesthetics can be helpful when administering treatment over a large surface area. The use of topical anesthetics can be beneficial and should be applied after application of the injection grid. Injectable local anesthetics can enhance patient comfort and may be used as a field block or a local block as appropriate. The authors recommend subcutaneous injection with 1-2% lidocaine with epinephrine 1:100,000 in the desired zone of treatment. Lower concentrations of lidocaine can be used if larger field blocks are planned, taking care to stay well below maximal safe dose for lidocaine. Applying ice is advised for at least 15 minutes before and after the procedure to improve comfort, minimize bruising, and reduce edema.
Injection Technique
A total of 0.2 cc is injected subcutaneously at each grid point (area-adjusted dose: 2 mg/cm2) using a 30- or 32-gauge 0.5 inch needle. Injections should be administered perpendicular to the surface of the skin with the needle positioned midway into the subcutaneous fat. Needle withdrawal during injection and superficial administration of ATX-101 should always be avoided to minimize the risk of dermal necrosis. A systematic approach to injection along the grid is prudent and becomes increasingly important the larger or more specific the area of treatment. No more than the maximum dose of 10 mL ATX-101 should be administered in a single treatment, regardless of the area being treated.
Safety Considerations
The safety and efficacy of ATX-101 injection for the treatment of submental fat has been rigorously studied. However, standards of practice and explicit indications relating to the use of ATX-101 in non-submental areas have not been established. Safe and effective application of ATX-101 beyond the submental region is predicated on a firm grasp of the pertinent anatomy, accurate identification of the treatment area, and skillful injection technique.
Clinicians should adhere to the following core principles:
- Only treat subcutaneous adipose tissue. Use skin-pinch techniques to confirm that the targeted fat is appropriately superficial.
- Avoid injecting in close proximity to critical nerves (e.g. motor and large sensory nerves). The cytolytic effects of ATX-101 can result in temporary demyelination and a period of nerve inflammation. Events of nerve injury and paresis typically resolve on the order of weeks to months, however, the effects of nerve injury (e.g. distorted facial expressions, asymmetry, numbness) cause undue distress to the patient.
- Avoid direct intravascular injection or injection in regions near critical anatomic structures.
- Do not exceed the recommended maximal dose per treatment session of 10 mL ATX-101. Using the recommended area-adjusted dose of 2 mg/cm2, a maximum of 50 injections (with 0.2 mL ATX-101 per injection) may be administered during a single treatment session. Based on the authors’ clinical experience, a maximum dose of 20 mL in a single session should be administered until the treating clinician feels comfortable with larger volume and larger dosage injections. Larger treatments can be staggered 24-hours apart.
- A maximum of 6 single treatments may be administered at intervals no less than 4 weeks apart. The number of prescribed treatment sessions will depend on the patient’s fat distribution, anatomy, and treatment goals.
- Patient selection is imperative throughout this process. Suitable patients should have excess adipose tissue and preserved skin elasticity in the area of treatment.
By following these core principles, ATX-101 can safely be administered beyond the submental region. Expansion of the submental safe zone was first described in adjacent areas of the neck. By restricting ATX-101 administration to the region 2 cm inferior to the margin of the mandible and anterior to the sternocleidomastoid, injury to regional nerves (i.e. the marginal mandibular nerve superiorly, and the great auricular and spinal accessory nerves laterally) can be avoided.
We will now explore the use of ATX-101 in the most commonly requested regions outside of the neck.
Regional Considerations
Anterior Trunk
Peri-Axillary Fat
Description & Considerations: Excess anterior peri-axillary fat (APAF),commonly known as “bra fat,” is an area of concern for many patients. Bulkiness in this area can cause aesthetic concerns as well as discomfort with clothing and brassieres. Excess APAF is a highly specific, focal collection of fat that lends itself well to treatment with ATX-101 without significant medication burden. During consultation, patients should be asked focused questions to determine the etiology of the fullness and to rule out the presence of ectopic breast tissue. Enhanced sensitivity or tenderness during the patient’s menstrual cycle suggests the presence of accessory breast tissue, which may reduce the effectiveness of the ATX-101 treatment.
Avoiding Critical Structures: The APAF treatment area is distinct and no critical structures traverse this region. Care should be taken to maintain a depth of injection above the underlying pectoralis; this requires careful attention in patients who have undergone prior breast augmentation surgery. Subglandular breast implants can be damaged with deep injection in a patient with attenuated overlying tissue. Additionally, injection directly into the axilla should be avoided. Maintaining superficial injection medial to lateral border of the pectoralis major ensures safe administration of ATX-101.
Pseudo-Gynecomastia
Description & Considerations: Among male patients, the accumulation of subcutaneous fat on the chest wall is one of the most common complaints. Even minimal amounts of fat can create a displeasing contour to the male chest. Pseudo-gynecomastia, in which fullness is secondary to fat accumulation and weight gain, should be distinguished from true gynecomastia caused by hypertrophy of glandular tissue. True gynecomastia is most appropriately treated with surgery, whereas pseudo-gynecomastia can be improved with injection adipocytolysis. The defined and limited area of lipodystrophy present in pseudo-gynecomastia makes this region suitable for treatment with ATX-101.
Avoiding Critical Structures: While no critical structures directly traverse the area of treatment in pseudo-gynecomastia, there are important considerations to discuss with the patient prior to treatment. Pain upon injection, swelling, and numbness are common side effects associated with ATX-101 injection to any region, however, these effects can be especially bothersome to male patients treated for pseudo-gynecomastia. Field blocks can be used to enhance patient comfort during injection. Patients should be counseled on the likelihood of prominent swelling post-treatment, as this can be aesthetically unpleasant and distressing for some patients. This swelling is self-limited, and typically resolves within 1-2 weeks; swelling may benefit from compression and the application of ice. Further, patients should be counseled on the possibility of self-limiting numbness and/or sensory changes involving the nipple; these effects can persist for several months.
Abdomen
Description & Considerations: While large-volume adiposity of the abdomen is best treated with surgical techniques, ATX-101 can be used for abdominal contouring in select patients. Patients with small, localized regions of stubborn fat, or patients with moderate subcutaneous fat seeking nonsurgical intervention, may benefit from injection adipocytolysis. Patients requesting treatment over a large surface area should be appropriately counseled on treatment cost as large volumes of ATX-101 will be required. Additionally, patients should be counseled on the likelihood of needing multiple treatment sessions and on the limitations of nonsurgical correction. Because the efficacy of ATX-101 treatment is dose-dependent, proper injection technique allows practitioners precise control over the final abdominal contour. Providers should be familiar with the general principles of abdominal contouring, as used in surgical liposuction. (Figure 1)
Figure 1. DCA injections to the abdomen.
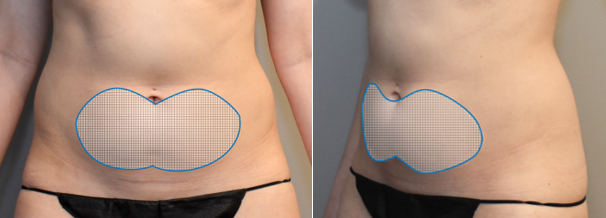
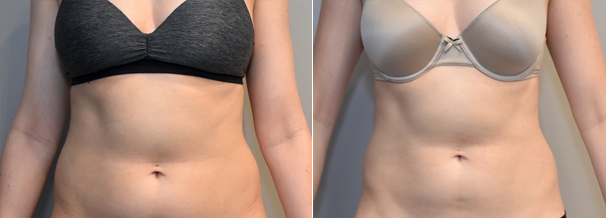
Fig. 1. A 33-year-old female patient was treated for excess fat of the lower abdomen. (A) Anterior and oblique views of the treatment area are shown. Patient is shown before treatment (B), and 3 months after her second treatment with injectable DCA to the lower abdomen (C). A total of 23 cc of DCA (10 mg/mL) was administered during the first treatment session. A total of 21 cc of DCA (10 mg/mL) was administered during second treatment session. The treatments sessions were spaced 8 weeks apart.
Avoiding Critical Structures: Injection above the abdominal musculature is safe with no nearby critical structures.
Posterior Trunk
Buffalo Hump
Description & Considerations: Dorso-cervical lipodystrophy, colloquially termed the buffalo hump, is a collection of firm, dense fat on the posterior upper back. While this region can be treated with ATX-101, it is the authors’ clinical experience that dorso-cervical lipodystrophy is better managed with other interventions. The fibrous nature of the fat in this region makes effective treatment with ATX-101 particularly challenging and the results of treatment are generally unsatisfactory. Additionally, fat accumulation in the dorso-cervical region may be secondary to other pathologies, including corticosteroid use or HIV-associated lipodystrophy. It is important to counsel patients on the limitations of ATX-101 for treating this region or avoid treating this region altogether.
Avoiding Critical Structures: Thesubcutaneous layer is relatively safe. However, aggressive or deep injection extending laterally increases the possibility of inadvertent injection to the trapezius muscle as it becomes superficial. For this reason, care should be taken when injecting laterally to observe skin pinch depth.
Posterior Back Rolls
Description & Considerations: The posterior back can be a challenging region to treat. This area includes fat overriding the latissimus dorsi and teres major/minor muscles and is composed of dense fibro-fatty and stomal tissue. As similarly noted during liposuction of the posterior back, the density of tissue in this region makes efficient debulking and sculpting difficult; the fat quality differs from that found on the abdomen, face, and extremities.
Physical exam is crucial in this area, as the etiology of deformity of posterior back rolls can be multifactorial. Many female patients requesting treatment here are dissatisfied with the appearance of overhang that occurs secondary to bra straps or fitted clothing. Any element of significant skin laxity contributing to the deformity, as is often the case, may not be adequately addressed with DCA alone. Physical examination to elucidate the etiology of the deformity remains paramount to appropriate patient selection.
Avoiding Critical Structures: This is a relative safe zone with no critical structures.
Flanks / Love Handles
Description & Considerations: At the transition from the abdomen to the back, the flank can be managed more similarly to the abdomen. The fat here is softer and less fibro-fatty, lending to ease of treatment by DCA or any other debulking modality. However, an important consideration is that there can be a deceivingly large amount of fat in this area. For individuals with substantial fullness (>5cm pinch test), multiple large-volume treatment sessions will be required to adequately treat this region. Prior to administering treatment, patients with significant fullness must be counseled about the need for multiple, large-volume injection sessions. So, while it is possible to treat patients with significant adiposity here, patients with mild or moderate adiposity of the flank are better candidates for treatment with DCA.
Avoiding Critical Structures: This is a relatively safe treatment zone with no critical structures.
Extremities
Upper Arms
Description & Considerations: The upper arms are a common area of lipodystrophy. This area is highly amenable to improvement with DCA as upper arm fat tends to be superficial. Additionally, surgical techniques can produce morbid scars, increasing the desirability of minimally-invasive treatment within this area. Typically, treatment proceeds along the posterior arm superior to the triceps muscle from the olecranon to the acromion process and includes fat overlying the deltoid muscle and lateral arm. (Figure 2)
Figure 2. DCA injections to the bilateral upper arms.
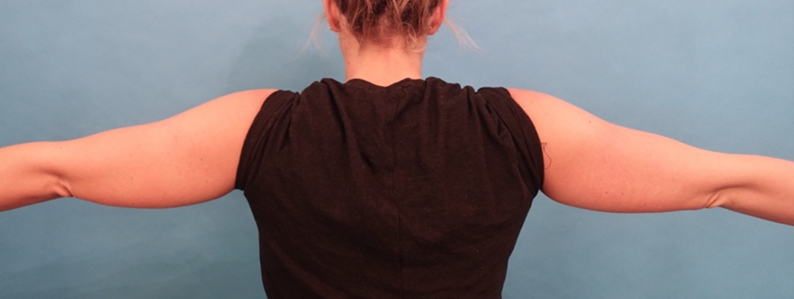


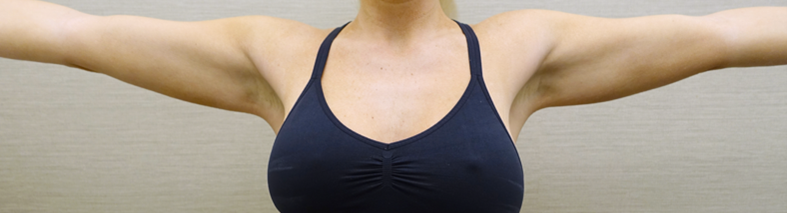
Fig. 2. A 29-year-old female patient is shown before treatment (A), and 4 months after her third treatment with injectable DCA to the bilateral upper arms. A total of 30 cc of DCA (10 mg/mL) was administered during the first treatment session (15 cc to the right upper arm; 15 cc to the left upper arm). A total of 34 cc of DCA (10 mg/mL) was administered during the second treatment session (16 cc to the right upper arm; 18 cc to the left upper arm). A total of 33 cc of DCA (10 mg/mL) was administered during the third treatment session (16 cc to the right upper arm; 17 cc to the left upper arm).
Avoiding Critical Structures: Special precaution must be taken when administering injections near the elbow. The ulnar nerve becomes superficial between the medial epicondyle and olecranon. Accordingly, if patients have a history of ulnar nerve decompression surgery, particularly an ulnar nerve transposition, care must be taken to avoid injection in this area. Important sensory structures include the medial antebrachial cutaneous nerve which traverses superficially in this same area proximally on the medial arm.
Hips & Outer Thighs **
Description & Considerations: The outer thigh is a commonly requested region of adiposity that is amenable to contouring and refinement with DCA injection. The fat here is soft without significant fibrous structure, lending to favorable treatment outcomes. Additionally, the outer thigh is prone to contour irregularities secondary to traditional liposuction techniques making injection adipocytolysis an appealing alternative. Patients should be assessed for degree of adiposity and patients with larger volumes of fat should be counseled on the need for increased product volume and multiple treatment sessions.(Figure 3)
Figure 3. DCA injections to the bilateral outer thighs.
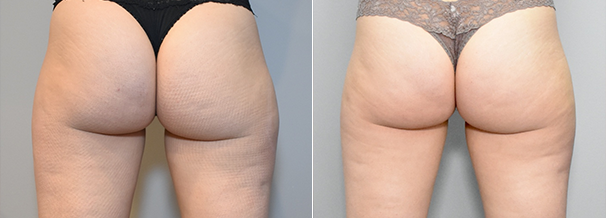
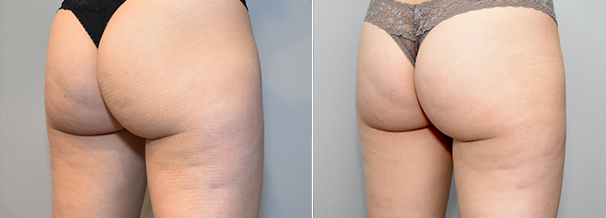
Fig. 3.A 31-year-old female patient is shown before treatment (A), and 2 months after her second treatment with injectable DCA to the bilateral outer thighs (B). A total of 28 cc of DCA (10 mg/mL) was administered during the first treatment session (15 cc to the right lateral thigh; 13 cc to the left lateral thigh). A total of 30 cc of DCA (10 mg/mL) was administered during the second treatment session (17 cc to the right lateral thigh; 13 cc to the left lateral thigh). The treatment sessions were spaced 4 weeks apart.
Avoiding Critical Structures: Zones of adherence including the iliotibial band and lateral gluteal depression should be identified, and aggressive treatment of these areas should be avoided.Due to the dependent nature of the lower extremity, this region is prone to prolonged edema and hemosiderin staining. Patients with lymphedema or venous insufficiency should not be treated with DCA if injecting below the groin. Healthy patients should be counseled to expect an extended period of edema when undergoing treatment to the lower extremities. Swelling can be partially mitigated by judicious use of compression garments.
Medial Thighs
Description & Considerations: The medial thigh is a common area of focal fatty accumulation, particularly in female patients. While aggressive treatment of this area may be tempting, practitioners should avoid overtreatment. The skin of the inner thigh is thin and inelastic and stretch marks are often present here, all of which can be exacerbated by aggressive debulking. Additionally, many patients have significant skin redundancy in this area, especially if they have had prior weight loss. For these reasons, proper patient selection is critical to favorable treatment outcomes. The ideal candidate will have good skin quality, minimal laxity, and focal fatty accumulation without the need for skin resection. Patients should be counseled that they will likely experience post-treatment numbness and swelling that can result in chaffing and abrasions of the medial thigh if unobserved during ambulation. Patient education and utilization of anti-chaffing ointments or powders may be helpful during the recovery process.
Avoiding Critical Structures: Recall the proximity of the femoral triangle in this area with its adjacent density of lymphatics. Deep injection should be avoided throughout this region.
Other / Miscellaneous
Mons Pubis
Description & Considerations: The mons can be favorably treated with DCA injection. Fullness of the mons region may occur primarily, or it may occur secondary to abdominoplasty. The typical area of injection extends from the upper limit of the pubic hairline to the anterior commissure of the labia majora (in women) or to base of the penis (in men). Patients should be counseled that this area can have prolonged swelling. Due to the dependent nature of this area, there can be associated labial or scrotal edema following DCA injection to the mons. This swelling does resolve spontaneously, however, it can be alarming to the uninformed patient; deliberate pre-procedure counseling is important.
Avoiding Critical Structures: The mons is a generally safe area for injection, however care must be taken to limit injections to the superficial fat. Injecting deeper fat planes risks temporary neuropraxia to the traversing sensory nerves to the genitalia. All patients should be counseled that temporary sensory changes to the genitalia are possible and that any sensory changes typically resolve alongside the resolution of swelling.
Conclusion
While large sample studies and clinical trials are warranted to establish the safety and efficacy of DCA outside the submental region, the authors’ clinical experience indicates non-submental applications of DCA to be effective and unaccompanied by significant adverse events. Considering the current cost of product, the most favorable applications for non-submental DCA injections involve small, focal accumulations of adipose tissue. Provided proper injection technique, prudent patient selection, and anatomic proficiency, injection adipocytolysis represents a viable nonsurgical alternative for addressing unwanted subcutaneous fat in regions of the body.
References
- ASPS National Clearinghouse of Plastic Surgery Procedural Statistics. 2019 Plastic Surgery Statistics Report. https://www.plasticsurgery.org/documents/News/Statistics/2019/plastic-surgery-statistics-full-report-2019.pdf. Accessed Oct 13, 2020.
- Full prescribing information: KYBELLA® (deoxycholic acid) injection. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206333s001lbl.pdf. Accessed Oct 13, 2020.
- National Center for Biotechnology Information. PubChem Compound Summary for CID 222528, Deoxycholic acid. https://pubchem.ncbi.nlm.nih.gov/compound/Deoxycholic-acid. Accessed Nov. 18, 2020.
- Salti, G. & Rauso, R. Comments on “Injection lipolysis with phosphatidylcholine and deoxycholate”. Aesthet Surg J 34, 639-640, doi:10.1177/1090820X14528506 (2014).
- Rotunda, A. M., Suzuki, H., Moy, R. L. & Kolodney, M. S. Detergent effects of sodium deoxycholate are a major feature of an injectable phosphatidylcholine formulation used for localized fat dissolution. Dermatol Surg 30, 1001-1008, doi:10.1111/j.1524-4725.2004.30305.x (2004).
- Pilsl, U. & Anderhuber, F. The chin and adjacent fat compartments. Dermatol Surg 36, 214-218, doi:10.1111/j.1524-4725.2009.01424.x (2010).
- Yagima Odo, M. E., Cuce, L. C., Odo, L. M. & Natrielli, A. Action of sodium deoxycholate on subcutaneous human tissue: local and systemic effects. Dermatol Surg 33, 178-188; discussion 188-179, doi:10.1111/j.1524-4725.2006.33036.x (2007).
