Introduction
There are currently more than 200 dermal fillers and volume enhancers available worldwide17—with a seemingly endless stream of new products being designed and manufactured every year. This is undoubtedly in response to the popularity of minimally-invasive aesthetic rejuvenation procedures3. As patients have become more familiar with different dermal fillers, they have also become more discerning consumers. A 2010 survey51 of more than 300 women found that the majority preferred gradual results that last two years over immediate results that last only six months and prioritized filler longevity over treatment cost. Fortunately, there is a safe and effective product already on the market that meets the public’s demand for a long-lasting dermal filler: Bellafill.
History
Following the FDA’s ban on liquid silicone in medical procedures in 1979, the use of injectable bovine collagen for soft tissue augmentation exploded. Just three years later a solution of soluble collagen became the first FDA-approved filler for soft tissue augmentation24,25. Bovine collagen thus became the gold standard to which all subsequent dermal fillers have been compared21. However, bovine collagen had limited longevity. This led to an interest in the development of a permanent, non-resorbable filler. The material with the least tissue reactivity turned out to be polymethylmethacrylate (PMMA)—which had been safely used in medical implants since World War II and had, by then, been widely used in neurosurgical, orthopedic, maxillofacial, ophthalmologic, and dental devices9,15.
Arteplast (1st Generation)
Arteplast was the original PMMA filler. It was developed by German plastic surgeon Gottfried Lemperle and was sold in Germany between 1989 and 1994. The original formulation was characterized by its gelatin carrier and the presence of PMMA microspheres measuring 20 to 40 microns in diameter.
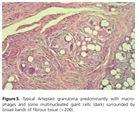
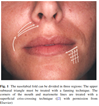
The first clinical trials began in 198938. Out of the original 187 volunteers and the additional 400 patients who received Arteplast until 1994, 2.5% of patients developed granulomas44 (Figure 539). The cause of granuloma formation was attributed to the adherence of PMMA nanoparticles to the surface of PMMA microspheres via static electrical charges that developed during the sieving process. The nanoparticle contaminants were small enough (smaller than 20 microns) to stimulate macrophages and be phagocytosed45. But the PMMA nanoparticles (being non-biodegradable) could not be broken down and removed—resulting in chronic granuloma formation32,33,44. Additionally, the rapid absorption of the gelatin carrier within the soft tissue led to clustering of the PMMA microspheres—causing palpable lumps in some patients30.
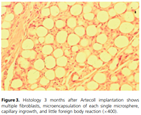
To address these issues, new sieving and washing processes were put into place to remove the offending PMMA nanoparticles and electrical charges and produce microspheres more uniform in size and with extremely smooth surfaces (Figure 239). A more viscous collagen solution was also designed as the carrier for the PMMA microspheres.
Artecoll (2nd Generation)
The new formulation was named Artecoll. It was manufactured by Rofil Medical International in the Netherlands and was available worldwide—except in the United States and Japan—from 1994 to 2006. Artecoll’s superior manufacturing process and improved formula led to a dramatic reduction in granuloma formation compared to its predecessor. Out of more than 200,000 patients treated with Artecoll, only 15 cases of granuloma formation were reported39. This represented a rate of less than 0.01%. Patient satisfaction rates were also favorable—with one study reporting 89% patient satisfaction30.
The initial FDA evaluation of Artecoll was a double-blinded, randomized controlled trial that was conducted at eight centers (four plastic surgery centers and four dermatology centers)7. The purpose of the study was to compare the safety and efficacy of Artecoll injections in the glabellar frown lines, nasolabial folds, radial upper lip lines, and corner-of-the-mouth (marionette) lines to the safety and efficacy of bovine collagen. At 12 months, significant and persistent wrinkle correction was noted in 87% of patients. Adverse events were uncommon. Based on these results, the FDA advisory panel recommended marketing approval of Artecoll on the condition that the bovine collagen—which had previously been sourced from the hides of calves from a closed herd in Germany—be made exclusively from hides of calves from a closed herd in the United States39. The improved product would be marketed under the name Artefill.
Artefill (3rd Generation)
Artefill (Artes Medical, San Diego, California) represents the third generation of injectable PMMA products. It was approved by the FDA in October 2006 for the correction of nasolabial folds. After Artes Medical filed for Chapter 7 bankruptcy in December 2008, Suneva Medical Inc. (San Diego, California) acquired Artes Medical. It now owns and distributes Artefill under the trade name Bellafill.
Bellafill (3rd Generation)
Bellafill (Suneva Medical Inc., San Diego, California) is a biphasic filler comprised of 20% nonresorbable PMMA microspheres suspended in 80% (mostly denatured) bovine collagen carrier gel. It is the culmination of third-generation microsphere technology. Extremely uniform, round, and smooth PMMA microspheres measuring 30-42 microns in diameter are produced during a meticulous and extensive purification process. This size range prevents phagocytosis by macrophages and the resultant giant cell formation and granulomatous inflammation36,45. The smoothness and uniformity of the individual microspheres also mitigate an effective inflammatory response: studies have shown that particles with rough and/or irregular surfaces are more likely to stimulate macrophages31. The PMMA microsphere’s resistance to phagocytosis means that they tend to become encapsulated with a thick fibrous capsule. It is this fibrous capsule around the PMMA microspheres that is responsible for the long-term tissue augmentation and the prevention of clustering of the PMMA microspheres37.
The use of Bellafill, however, has been less extensive compared with hyaluronic acid fillers. This likely stems from clinicians’ unfamiliarity with the product, concerns over product permanency, the necessity of skin testing, and the risk profile associated with earlier generations of PMMA-collagen products (i.e., Arteplast, Artecoll). Yet Bellafill is considered extremely safe—with a risk profile comparable to hyaluronic acid fillers. A 2019 report of 12 years of post-market surveillance data (i.e., 754,229 syringes) found that the overall incidence of complications was just 0.11%27. The three most common adverse events were lumps/bumps (0.04%), nodules (0.02%), and swelling (0.018%)—all of which are typically mild, transient, and/or easily treated. Granulomas were even rarer (0.011%). The safety profile and long-lasting effect are likely responsible for Bellafill’s high patient satisfaction rate of 82% at six months and 84% at five years6.
Biocompatibility
Animal experiments have demonstrated that the biocompatibility of Bellafill depends on both the uniform 30-42 micron diameter and the smooth surface of the PMMA microspheres35,37. These properties are responsible for the low incidence of granuloma formation. All other long-lasting synthetic dermal fillers (e.g., Teflon, silicone) have particles with an irregular surface and result in foreign body granulomas due to “frustrated macrophages”12,35. In contrast, the large, smooth, electrically-neutral surface of the PMMA microspheres do not induce a chronic inflammatory reaction because the particles cannot be phagocytosed by macrophages. Instead, the PMMA microspheres are encapsulated by fibrous tissue and become “incorporated” into the host’s soft tissue.
Histology
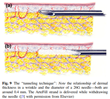
When Bellafill is initially injected, the collagen carrier gel provides immediate mechanical filling of the volume-deficient treatment area. Within one to four months, the collagen gel is rapidly broken down by collagenases and phagocytized by macrophages. As with collagen-only dermal fillers, the removal of Bellafill’s collagen carrier does not stimulate the production of new collagen fibers23,35. During the same period of time, new collagen is deposited by the host to encapsulate the PMMA microspheres (Figure 339). Since the PMMA microspheres cannot be broken down by enzymes and are too large to be phagocytosed by macrophages, they act as a scaffold and a stimulus for constant connective tissue production2,45.
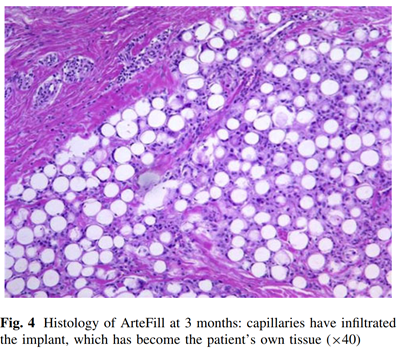
These processes are evident in vivo within one week of injection—with the infiltration of macrophages and ingrowth of tissue proteoglycans (signals of new tissue production) in and among the PMMA microspheres49. By one month, type I collagen becomes one of the dominant collagen subtypes as it progressively accumulates and develops a mature fibrillary structure49. These new collagen deposits surround each individual PMMA microsphere and increase the spaces between them35. At three months, the original bovine collagen has resorbed, and there is complete encapsulation of the PMMA microspheres by fibroblasts and collagen fibers34. Macrophages and other inflammatory cells are rare. By seven months, the new collagen fibers around the PMMA microspheres are indistinguishable from the adjacent collagen fibers46. At ten years, intact PMMA microspheres are fully integrated into the surrounding connective tissue34. Mature collagen fibers, a fully intact capillary system, and a steady turnover of fibroblasts and macrophages are all present34.
Mechanism of Action
After Bellafill is injected, immediate mechanical filling and wrinkle correction is achieved by the collagen gel component. As the collagen carrier is phagocytosed by macrophages (over a period of one to four months), encapsulation of the PMMA microspheres by endogenously-derived connective tissue (i.e., neocollagenesis) is responsible for persistent correction and prevents migration and clustering of the PMMA microspheres4 (Figure 128). Based on experience with this dermal filler in Europe, the aesthetic effects can last for more than 10 years34.
Packaging
Each Bellafill package contains a single prefilled syringe with a 0.8-mL fill volume. Each syringe consists of PMMA microspheres (about 6 million microspheres per 1 mL of product) evenly suspended in a water-based carrier gel composed of 3.5% bovine collagen, 2.7% phosphate buffer, 0.9% sodium chloride, 0.3% lidocaine hydrochloride, and 92.6% buffered, isotonic water. The bovine collagen is derived from an isolated herd of US-bred calves monitored according to FDA and USDA guidelines. Moreover, the collagen gel and the PMMA microspheres are both manufactured and then combined in a dedicated facility in the United States. By volume, Bellafill consists of 20% PMMA microspheres and 80% bovine collagen carrier gel.
Bellafill should be stored in a refrigerator at standard temperatures (2–8°C) until it is ready to be used52. It should be allowed to come to room temperature before injection to ease the flow of the filler through the syringe and needle.
Allergy Testing
Because of the presence of bovine collagen in Bellafill, patients must be tested for bovine collagen allergy with the Bellafill Skin Test. Each Bellafill Skin Test package has either 2 or 5 syringes. Each syringe contains 0.3 mL of purified collagen gel (which is identical to the collagen gel found in Bellafill).
At least four weeks prior to treatment, 0.1 mL of the Bellafill Skin Test product is injected intradermally into the volar forearm53. A positive skin test response consists of erythema (of any degree), induration, tenderness, and/or swelling with or without pruritus appearing within 24 hours following injection and lasting more than 24 hours or appearing more than 24 hours following injection. An equivocal response is one in which there is no localized skin reaction but there is a systemic reaction (e.g., rash, arthralgias, myalgias) at any time during the 4-week observation period. If an equivocal response is observed, a second skin test must be performed on the opposite arm with an additional 4-week observation period. Patients showing a positive skin test or two equivocal skin tests are not candidates for treatment. Clinicians should also keep in mind that a negative response on the Bellafill Skin Test does not preclude the possibility of a patient becoming sensitized to the bovine collagen carrier gel after multiple injections or developing a delayed hypersensitivity response to the bovine collagen carrier gel following the initial treatment exposure20,47.
Bellafill Skin Test syringes should be stored in a refrigerator at standard temperatures (2–8°C) until they are ready to be used53. Syringes should be allowed to come to room temperature before injection to ease the flow of the filler through the syringe and needle.
Patient Selection and Indications
Bellafill is the only FDA-approved permanent injectable filler available in the United States. It was originally approved in 2006 (under the name Artefill) for the treatment of the nasolabial folds. In 2014, it gained FDA-approval for correction of moderate-to-severe, atrophic, distensible facial acne scars on the cheeks of patients over the age of 21 years. It is also used off-label for horizontal forehead lines, tear trough deformity, malar augmentation, marionette lines, and chin augmentation (Table 1). The best candidates for treatment with Bellafill have deep, well-defined wrinkles, little excess skin, and relatively thick skin in the treatment area. Thin skin may lead to beading, palpability, and visibility of the filler5,11.
Bellafill is contraindicated in patients who have positive or equivocal results to the Bellafill Skin Test; patients who have a history of allergies to bovine collagen products; patients showing an anti-bovine collagen IgG level outside the normal range; patients who have severe allergies (as indicated by a history of multiple severe allergies or anaphylaxis); patients who have a known lidocaine hypersensitivity; and patients who have known susceptibility to keloid formation or hypertrophic scarring.
| Approved & Off-Label Aesthetic Indications | |
| Horizontal Forehead Lines | Facial Wasting |
| Tear Trough Deformity | Cheek Depressions |
| Malar Augmentation | Acne Scars |
| Nasolabial Folds | Hand Rejuvenation |
| Temporal Hollowing | |
| Marionette Lines | |
| Prejowl Sulcus | |
| Chin Augmentation |
Injection Technique
The method for injecting Bellafill is more technique-sensitive than that for temporary fillers. Significant practice is often necessary before a clinician develops a feel for the correct injection pressure, correct injection level, and beveling of the injection needle. It also takes time before a clinician feels comfortable with the irreversibility of this dermal filler. Thus it is recommended that clinicians start by treating areas with thick skin, deeper creases, and/or and/or greater soft tissue deficiencies.
Even though Bellafill contains 0.3% lidocaine to alleviate discomfort during injection, other anesthetic measures should be taken to minimize pain. One popular method is the application of a topical anesthetic to the skin 45 to 60 minutes prior to treatment. Some clinicians also add a field block when deeper injections are planned. The use of a cold compress immediately prior to and after injection is common as well.
Prior to injection of Bellafill, it is important to allow the syringe to thaw for 30 to 45 minutes. Until the product comes to room temperature, it is very viscous and difficult to inject. Once adequately thawed, Bellafill should be injected directly into the treatment area with a 26-gauge 5/8-inch-long needle52. Because Bellafill has greater viscosity than other dermal fillers, higher pressures are required to extrude Bellafill from the syringe and needle42. Clinicians also must generate consistently high pressure throughout the injection to prevent uneven or bolus deposition of Bellafill in the treatment area. The use of longer and/or narrower needles is not recommended.
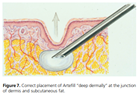
Bellafill should be injected at the dermal-subcutaneous junction or into deeper tissues (Figure 739). Placement more superficially (i.e., intradermally) can lead to permanent skin color or surface texture changes. Beading, ridging, palpability, visibility, and nodule formation (which can only be reversed by surgical excision) have all been reported after injection into the superficial dermis and mid-dermis18,48. Thus it is preferable to inject more deeply than superficially—even at the risk of wasting filler material. A downward-facing bevel helps to minimize unwanted deposition of the filler in a too-superficial location.
The ideal injection technique for Bellafill depends on the planned depth of placement. Uniform distribution in the subdermal plane is best accomplished using the tunneling and retrograde linear threading technique (Figure 934): the advancing needle creates a tunnel which is then filled with one strand of Bellafill as the needle is withdrawn. Some clinicians even recommend passing the needle two or three times prior to placement of any small subdermal strands14. This is especially useful when treating scars: fanning the needle tip across the scar bed several times in a subcision fashion creates space for the filler to be injected26. Deeper injections (e.g., supraperiosteal placement for chin augmentation) can be accomplished either with the tunneling and retrograde linear threading technique or the serial puncture technique—depending on the treatment area and clinician preference.
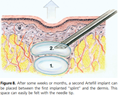
The volume of product to be injected depends on the depth and size of the wrinkle or the amount of soft-tissue deficiency. But, since removal of Bellafill is difficult without excision, treatments should always be conservative. Overcorrection should be avoided. Patients should be counseled that at least two injection sessions are required to achieve final correction. During the first treatment session, it is best to aim for partial correction. Patients should then be evaluated 4 to 6 weeks later to assess the need for additional augmentation—as 50% of patients will require a second treatment to achieve full correction34 (Figure 839). Small touch-up treatments may also need to be performed periodically depending upon the treatment site, injection depth, dynamics of the corrected site, and host’s response to previous treatment(s)21. It is generally agreed that gradual correction of wrinkles and soft tissue defects permits a more natural-looking result22.
Specific Treatment Areas
Nasolabial Folds
The nasolabial fold can be divided into three separate treatment areas: the upper (subnasal triangle), middle, and lower. Injection should begin at the superior aspect of the fold. In the upper region, the needle either should be inserted directly onto the periosteum (slightly lateral to the nasal ala) or fanned in the subdermal plane to fill the deep subnasal triangle (Figure 140). Regardless of the technique, it is important to stay slightly medial to the crease. The middle and lower regions are best supported by at least two to three strands of Bellafill injected parallel and approximately 1-2 mm medial to the crease. Care must be taken to avoid injecting too superficially (i.e., intradermally) to prevent ridge formation (Figure 340). Also, since Bellafill is a viscous paste during the first three days after implantation, Bellafill may be moved laterally or dislodged into the deeper layers by pronounced facial muscle movement. Patients must be advised to limit facial muscle movement during this time period. At least two treatments are usually necessary.
Horizontal Forehead Lines
Transverse forehead rhytides respond well to subdermal placement of Bellafill parallel and directly underneath the lines. Care must be taken so that the grey of the needle is not visible through the wrinkle line during injection. Superficial (i.e., intradermal) injection may result in ridge formation or formation of a string of palpable nodules (similar to what is seen in Figure 340). Two to three sessions are often required for deeper rhytides.
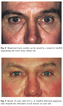
Tear Trough & Malar Augmentation
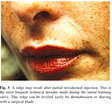
Patients with dark rings around the lower rims of their eyes are ideal candidates for long-term correction with Bellafill (Figures 8 & 940). Supraperiosteal injection of two to three strands of Bellafill along the length of the infraorbital rim is usually adequate to smooth the transition between the lower eyelid and cheek. Whether the serial puncture or linear threading technique is used, the patient should be injected while in the upright position. For the linear threading technique, the needle is inserted lateral-to-medial just inferior to the deepest aspect of the tear trough. The bone is felt with the tip of the needle to confirm proper placement. Care must be taken to avoid injecting above the level of the orbital rim. After injecting the product, avoid applying pressure to the syringe during withdrawal through the muscle: intramuscular deposition of Bellafill may lead to nodule formation.
Augmentation of the adjacent malar region is frequently done in combination with correction of the tear trough. The needle is inserted slightly lateral and slightly superior to the bony malar eminence. The needle is then fanned radially, and Bellafill is injected supraperiosteally. As the injections move laterally and inferiorly away from the lid-cheek junction, product placement may be transitioned to a more subcutaneous plane.
Marionette Lines
Rejuvenation of the perioral region can be more difficult than other areas because there is minimal subdermal fat between the skin and orbicularis oris muscle. This makes accurate implantation at the dermal-subdermal junction challenging. Often there are not only deep lines extending from the oral commissures toward the mandibular borders (i.e., marionette lines) but also volume deficiencies in the surrounding areas in many patients.
Treatment should begin along the white roll at the corner of the mouth. Multiple vertical and horizontal subdermal threads of Bellafill placed in a crosshatch or fanning pattern support the lip commissure and treat both the crease and any adjacent volume deficiency (Figure 140). Care must be taken to confirm appropriate filler placement to prevent ridge formation (from intradermal implantation) and nodule formation (from intramuscular implantation). At least two treatments are generally required.
Prejowl Sulcus
If a surgical facelift is not indicated, the prejowl sulcus may be corrected with supraperiosteal injection into the deepest part of the depression—with the goal of raising the skin and leveling the margin of the mandible. If necessary, the injection can extend from the anterior margin of the jowl toward the midline of the chin to help restore a more youthful appearance.
Acne Scars
Bellafill is very effective for the correction of mature, mildly depressed, distensible acne scars and is currently the only permanent treatment option approved by the FDA. (Fresh immature scars should never be treated with Bellafill as their appearance may worsen.) Mature acne scars may be filled either obliquely from about 5-10 mm away or via serial injection of micro-aliquots delivered perpendicular(ly downward) directly into the center of the scar. If “ice-pick” acne scars are present, subcision (e.g., using an 18-gauge needle) or punching and suturing must first be performed; subsequent filling of the wound cavity with Bellafill may be performed one week later (after the swelling has subsided and the incision has healed).
Complications / Adverse Events
Technical Errors
A primary concern of clinicians is the type and frequency of adverse events. Fortunately (or unfortunately) complications arising from Bellafill injections are often related to injection technique. Clinicians who fail to learn the correct injection pressure, injection depth, injection volume, and beveling of the injection needle (etc) are more likely to experience adverse events.
The most common of these mistakes is inappropriate injection depth. If Bellafill is implanted too superficially, patients may experience persistent redness and itching—which usually respond to topical corticosteroids or intradermal corticosteroid injections. Ridging, beading, and intradermal nodules may also occur a few weeks after too-superficial implantation. Use of Bellafill in areas of thin skin (e.g., in and around the lips, around the eyes) and repetitive facial muscle movement may further exacerbate the palpability and visibility of the filler1. Ridges and nodules not shining through the skin are best treated with dermabrasion or tangential shaving to make the skin surface even18,48. Neither of these treatments will create a noticeable scar since both the exposed collagen carrier gel of Bellafill and the fibrous capsules around the PMMA microspheres will epithelialize like normal abraded skin. Excision and suturing is seldom necessary. But, if excision is indicated, all of the filler should be removed to avoid continued fibroblast stimulation and secondary hypertrophic scarring39.
Improper placement of Bellafill too deeply (e.g., in the subcutaneous tissue) will result in no filling effect on the crease. Displacement of filler into the deeper subcutaneous tissue may also occur following strong facial muscle movement during the first three days after injection or repetitive facial muscle movements over several years—especially if the filler was already in the superficial subcutaneous fat. If any of these occur, another injection (on top of the prior implant) is reasonable. If Bellafill is implanted directly into muscle, the filler may become dislodged into the adjacent tissues or become palpable nodules. These can be softened with intralesional corticosteroid injections. If a nodule is palpable intraorally, excision of the entire lesion may be necessary.
Another common complication is uneven distribution of Bellafill during injection. The resulting “string of pearls” causes inconsistent correction of the wrinkle or contour irregularities. A second treatment with placement of filler into the gaps is necessary to achieve correction of this defect.
Hypertrophic Scarring
Hypertrophic scarring has also been reported following treatment with Bellafill39. It likely results from superficial implantation, persistent stimulation of fibroblast activity by the PMMA microspheres, and subsequent fibrosis. Intralesional corticosteroids are the treatment of choice.
Telangiectasia
Telangiectasias may sometimes develop in areas with thin skin48. If they do not disappear spontaneously within six months, treatment with a laser or Intense Pulsed Light may be required.
Swelling, Erythema, & Bruising
Swelling and erythema immediately after injection of Bellafill are not uncommon. These are usually mild and transient. Ecchymosis occasionally occurs and is technique-dependent. Symptomatic or supportive treatment includes cold compresses and elevation.
Hypersensitivity Reactions
Bellafill’s PMMA microspheres are non-allergenic, but hypersensitivity reactions to Bellafill’s collagen carrier gel may occur. Compared to previous generations of PMMA-collagen fillers and bovine collagen preparations, Bellafill is much less likely to induce a hypersensitivity reaction. This is because the manufacturing process includes enzymatic removal of most of the allergenic telopeptides present at the end of native collagen polymers19. This low allergy rate (0.2%) is much lower than the 3-4% incidence of allergic reactions seen with bovine collagen fillers and only slightly higher than that of HA fillers at 0.15%8,10,16,34,43,50.
Clinicians should also keep in mind that a negative response on the Bellafill Skin Test does not preclude the possibility of a patient becoming sensitized to the bovine collagen carrier gel after multiple injections or developing a delayed hypersensitivity response to the bovine collagen carrier gel following the initial treatment exposure20,47.
Granuloma Formation
Granulomas are the result of an overly aggressive response of the body’s inflammatory system—especially cell-mediated immunity. In the case of PMMA-collagen, the PMMA microspheres become surrounded by nodular or diffuse infiltrates composed of epitheliod histiocytes, multinucleated giant cells, lymphocytes and eosinophils41,48. This can occur weeks, months, or even years after treatment and may be difficult to distinguish from nodules or an uneven distribution of filler product13.
Early formulations of PMMA-collagen were associated with an unacceptably high incidence of granuloma formation. Bellafill, however, appears to have avoided this outcome thanks to advances in the manufacturing process and an improved formula. A 2019 report of 12 years of post-market surveillance data (i.e., 754,229 syringes) found that the incidence of granulomas with Bellafill was just 0.011%27. This is consistent with the incidence of granuloma formation for other dermal fillers31.
Most filler-induced granulomas may be treated successfully with intralesional corticosteroid injections. A 1:1 mixture of lidocaine and triamcinolone (up to 20 mg/mL) or betamethasone (up to 5 mg/mL) can be safely injected through a 1-mL syringe and a 30-gauge needle directly into the nodule29. Any corticosteroids injected in the surrounding soft tissue (especially fat) may cause temporary—or, in rare instances, permanent—atrophy. If atrophy does occur, revolumization with a temporary filler will disguise the depression until the body naturally recovers in 3-12 months29.
If intralesional corticosteroid injections are unsuccessful, excision of the entire implant will be necessary.
Conclusion
Dermal fillers have become a mainstay in the arsenal of aesthetic rejuvenation procedures. To achieve optimal outcomes, a thorough understanding of fillers is necessary. Despite being around for over a decade, Bellafill is often overlooked. Unlike other dermal fillers, Bellafill stimulates endogenous collagen production and becomes fully integrated into the host connective tissue. In short, Bellafill is an excellent dermal filler for long-lasting improvement of facial wrinkles and furrows, soft tissue contour deficiencies, and acne scars.
Citations
- Ahn CS, Rao BK. The life cycles and biological end pathways of dermal fillers. J. Cosmet. Dermatol. 2014 Sep;13(3):212–223. doi:10.1111/jocd.12100
- Ali U, Karim KJBtA, Buang NA. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polym. Rev. 2015 Oct 2;55(4):678–705. doi:10.1080/15583724.2015.1031377
- American Society of Plastic Surgeons. 2018 Plastic Surgery Statistics Report [Internet]. [cited 2020 Jun 21]. Available from: https://www.plasticsurgery.org/documents/News/Statistics/2018/plastic-surgery-statistics-full-report-2018.pdf
- Carruthers A, Carruthers JDA. Polymethylmethacrylate Microspheres/Collagen as a Tissue Augmenting Agent: Personal Experience over 5 Years. Dermatol. Surg. 2005 Nov;31:1561–1565. doi:10.2310/6350.2005.31242
- Carruthers JDA, Carruthers A. Facial Sculpting and Tissue Augmentation. Dermatol. Surg. 2005 Nov;31:1604–1612. doi:10.2310/6350.2005.31248
- Cohen S, Dover J, Monheit G, Narins R, Sadick N, Werschler WP, et al. Five-Year Safety and Satisfaction Study of PMMA–Collagen in the Correction of Nasolabial Folds: Dermatol. Surg. 2015 Dec;41:S302–S313. doi:10.1097/DSS.0000000000000542
- Cohen SR, Holmes RE. Artecoll: A Long-Lasting Injectable Wrinkle Filler Material: Report of a Controlled, Randomized, Multicenter Clinical Trial of 251 Subjects: Plast. Reconstr. Surg. 2004 Sep;114(4):964–976. doi:10.1097/01.PRS.0000133169.16467.5F
- Delustro F, Mackinnon V, Swanson NA. Immunology of Injectable Collagen in Human Subjects. J. Dermatol. Surg. Oncol. 1988 Jul;14:49–55. doi:10.1111/j.1524-4725.1988.tb04040.x
- DiMaio FR. The science of bone cement: a historical review. Orthopedics. 2002 Dec;25(12):1399–1407; quiz 1408–1409.
- Elson ML. The Role of Skin Testing in the Use of Collagen Injectable Materials. J. Dermatol. Surg. Oncol. 1989 Mar;15(3):301–303. doi:10.1111/j.1524-4725.1989.tb03163.x
- Eppley BL, Dadvand B. Injectable Soft-Tissue Fillers: Clinical Overview: Plast. Reconstr. Surg. 2006 Sep;118(4):98e–106e. doi:10.1097/01.prs.0000232436.91409.30
- Eppley BL, Summerlin D-J, Prevel CD, Michael Sadove A. Effects of a positively charged biomaterial for dermal and subcutaneous augmentation. Aesthetic Plast. Surg. 1994;18(4):413–416. doi:10.1007/BF00451350
- Fischer J, Metzler G, Schaller M. Cosmetic Permanent Fillers for Soft Tissue Augmentation: A New Contraindication for Interferon Therapies. Arch. Dermatol. [Internet]. 2007 Apr 1 [cited 2020 Jul 1];143(4). Available from: http://archderm.jamanetwork.com/article.aspx?doi=10.1001/archderm.143.4.507doi:10.1001/archderm.143.4.507
- Fitzgerald R, Graivier MH, Kane M, Lorenc ZP, Vleggaar D, Werschler WmP, et al. Nonsurgical Modalities to Treat the Aging Face. Aesthet. Surg. J. 2010 Jul 1;30(1_Supplement):31S-35S. doi:10.1177/1090820X10373361
- Frazer RQ, Byron RT, Osborne PB, West KP. PMMA: An Essential Material in Medicine and Dentistry. J. Long. Term Eff. Med. Implants. 2005;15(6):629–639. doi:10.1615/JLongTermEffMedImplants.v15.i6.60
- Friedman PM, Mafong EA, Kauvar ANB, Geronemus RG. Safety Data of Injectable Nonanimal Stabilized Hyaluronic Acid Gel for Soft Tissue Augmentation. Dermatol. Surg. 2002 Jun;28(6):491–494. doi:10.1046/j.1524-4725.2002.01251.x
- Glogau RG. Fillers: From the Past to the Future. Semin. Cutan. Med. Surg. 2012 Jun;31(2):78–87. doi:10.1016/j.sder.2012.03.004
- Goldberg DJ. Breakthroughs in US dermal fillers for facial soft-tissue augmentation. J. Cosmet. Laser Ther. 2009 Jan;11(4):240–247. doi:10.3109/14764170903341731
- Graivier MH, Bass LM, Lorenc ZP, Fitzgerald R, Goldberg DJ, Lemperle G. Differentiating Nonpermanent Injectable Fillers: Prevention and Treatment of Filler Complications. Aesthet. Surg. J. 2018 Apr 6;38(suppl_1):S29–S40. doi:10.1093/asj/sjy032
- Jacob CI, Dover JS, Kaminer MS. Acne scarring: A classification system and review of treatment options. J. Am. Acad. Dermatol. 2001 Jul;45(1):109–117. doi:10.1067/mjd.2001.113451
- Jones DH. Semipermanent and Permanent Injectable Fillers. Dermatol. Clin. 2009 Oct;27(4):433–444. doi:10.1016/j.det.2009.08.003
- Jordan DR. Soft-tissue fillers for wrinkles, folds and volume augmentation. Can. J. Ophthalmol. 2003 Jun;38(4):285–288. doi:10.1016/S0008-4182(03)80093-5
- Kligman AM, Armstrong RC. Histologic Response to Intradermal Zyderm and Zyplast (Glutaraldehyde Cross-Linked) Collagen in Humans. J. Dermatol. Surg. Oncol. 1986 Apr;12(4):351–357. doi:10.1111/j.1524-4725.1986.tb01920.x
- Knapp TR, Kaplan EN, Daniels JR. Injectable collagen for soft tissue augmentation. Plast. Reconstr. Surg. 1977 Sep;60(3):398–405.
- Knapp TR, Luck E, Daniels JR. Behavior of solubilized collagen as a bioimplant. J. Surg. Res. 1977 Aug;23(2):96–105. doi:10.1016/0022-4804(77)90196-2
- Lee JC, Lorenc ZP. Synthetic Fillers for Facial Rejuvenation. Clin. Plast. Surg. 2016 Jul;43(3):497–503. doi:10.1016/j.cps.2016.03.002
- Lehman A, Pilcher B, Roberts WE, Schlesinger TE, Vachon G. Postmarket Experience of Polymethylmethacrylate–Collagen Gel Dermal Filler. Dermatol. Surg. 2020 Aug;46(8):1086–1091. doi:10.1097/DSS.0000000000002222
- Lemperle G, de Fazio S, Nicolau P. ArteFill: A Third-Generation Permanent Dermal Filler and Tissue Stimulator. Clin. Plast. Surg. 2006 Oct;33(4):551–565. doi:10.1016/j.cps.2006.09.004
- Lemperle G, Gauthier-Hazan N. Foreign Body Granulomas after All Injectable Dermal Fillers: Part 2. Treatment Options: Plast. Reconstr. Surg. 2009 Jun;123(6):1864–1873. doi:10.1097/PRS.0b013e3181858f4f
- Lemperle G, Gauthier-Hazan N, Lemperle M. PMMA-Microspheres (Artecoll) for Long-Lasting Correction of Wrinkles: Refinements and Statistical Results. Aesthetic Plast. Surg. 1998 Sep 1;22(5):356–365. doi:10.1007/s002669900217
- Lemperle G, Gauthier-Hazan N, Wolters M, Eisemann-Klein M, Zimmermann U, Duffy DM. Foreign Body Granulomas after All Injectable Dermal Fillers: Part 1. Possible Causes: Plast. Reconstr. Surg. 2009 Jun;123(6):1842–1863. doi:10.1097/PRS.0b013e31818236d7
- Lemperle G, Hazan-Gaúthier N, Lemperle M. PMMA Microspheres (Artecoll) for Skin and Soft-Tissue Augmentation. Part II: Clinical Investigations. Plast. Reconstr. Surg. 1995 Sep;96(3):627–634. doi:10.1097/00006534-199509000-00015
- Lemperle G, Holmes R, Larson F. Granuloma formation and electrical surface charges after Bioplastique and Arteplast implantation. Aesthetic Plast Surg. 2000;24:74–75.
- Lemperle G, Knapp TR, Sadick NS, Lemperle SM. ArteFill® Permanent Injectable for Soft Tissue Augmentation: I. Mechanism of Action and Injection Techniques. Aesthetic Plast. Surg. 2010 Jun;34(3):264–272. doi:10.1007/s00266-009-9413-1
- Lemperle G, Morhenn V, Charrier U. Human Histology and Persistence of Various Injectable Filler Substances for Soft Tissue Augmentation. Aesthetic Plast. Surg. 2003 Oct 1;27(5):354–366. doi:10.1007/s00266-003-3022-1
- Lemperle G, Morhenn VB, Pestonjamasp V, Gallo RL. Migration Studies and Histology of Injectable Microspheres of Different Sizes in Mice: Plast. Reconstr. Surg. 2004 Apr;113(5):1380–1390. doi:10.1097/01.PRS.0000112764.22839.7A
- Lemperle G, Ott H, Charrier U, Hecker J, Lemperle M. PMMA microspheres for intradermal implantation. I. Animal research. Ann Plast Surg. 1991;26:57–63.
- Lemperle G, Pietz R, Lemperle M. First clinical experiences with Arteplast (PMMA microspheres) injected beneath wrinkles and dermal defects. In: Hinderer UT, ed Plastic Surgery (vol. 2). Amsterdam: Elsevier; 1992. p. 539–541.
- Lemperle G, Romano JJ, Busso M. Soft Tissue Augmentation With Artecoll: 10-Year History,Indications, Techniques, and Complications. Dermatol. Surg. 2003 Jun;29(6):573–587. doi:10.1046/j.1524-4725.2003.29140.x
- Lemperle G, Sadick NS, Knapp TR, Lemperle SM. ArteFill® Permanent Injectable for Soft Tissue Augmentation: II. Indications and Applications. Aesthetic Plast. Surg. 2010 Jun;34(3):273–286. doi:10.1007/s00266-009-9414-0
- Lombardi T, Samson J, Plantier F, Husson C, Kuffer R. Orofacial granulomas after injection of cosmetic fillers. Histopathologic and clinical study of 11 cases. J. Oral Pathol. Med. 2004 Feb;33(2):115–120. doi:10.1111/j.1600-0714.2004.00194.x
- Lorenc ZP, McArthur T, Patael N, Pilcher B, Vachon G. Rheological Analysis of Polymethylmethacrylate-Collagen Injectable Filler and Comparison to Semi-Permanent and Temporary Fillers. Submitted for Publication.
- Lupton JR, Alster TS. Cutaneous Hypersensitivity Reaction to Injectable Hyaluronic Acid Gel. Dermatol. Surg. 2000 Feb;26(2):135–137. doi:10.1046/j.1524-4725.2000.99202.x
- Mang W, Sawatzki K. Fremdkoerperreaktion nach Implantation von PMMA (Polymethylmethacrylat) zur Weichteilaugmentation. Z Hautkrht, H+G. 1998;73:42.
- Morhenn VB, Lemperle G, Gallo RL. Phagocytosis of Different Particulate Dermal Filler Substances by Human Macrophages and Skin Cells. Dermatol. Surg. 2002 Jun;28(6):484–490. doi:10.1046/j.1524-4725.2002.01273.x
- Nicolau PJ. Long-Lasting and Permanent Fillers: Biomaterial Influence over Host Tissue Response: Plast. Reconstr. Surg. 2007 Jun;119(7):2271–2286. doi:10.1097/01.prs.0000260710.30934.a1
- Pessa JE, Peterson ML, Thompson JW, Cohran SC, Garza JR. Pyriform Augmentation as an Ancillary Procedure in Facial Rejuvenation Surgery: Plast. Reconstr. Surg. 1999 Feb;103(2):683–686. doi:10.1097/00006534-199902000-00050
- Requena L, Requena C, Christensen L, Zimmermann US, Kutzner H, Cerroni L. Adverse reactions to injectable soft tissue fillers. J. Am. Acad. Dermatol. 2011 Jan;64(1):1–34. doi:10.1016/j.jaad.2010.02.064
- Ronan SJ, Eaton L, Lehman A, Pilcher B, Erickson CP. Histologic Characterization of Polymethylmethacrylate Dermal Filler Biostimulatory Properties in Human Skin: Dermatol. Surg. 2019 Dec;45(12):1580–1584. doi:10.1097/DSS.0000000000001877
- Stegman SJ, Chu S, Armstrong RC. Adverse Reactions to Bovine Collagen Implant: Clinica1 and Histologic Features. J. Dermatol. Surg. Oncol. 1988 Jul;14:39–48. doi:10.1111/j.1524-4725.1988.tb04039.x
- Weinkle S, Lupo M. Attitudes, awareness, and usage of medical antiaging treatments: results of a patient survey. J. Clin. Aesthetic Dermatol. 2010 Sep;3(9):30–33.
- Suneva Medical Inc. Bellafill Instructions for Use [Internet]. [cited 2020 Jun 20];Available from: https://bellafill.com/wp-content/uploads/2018/12/7251Rev00_Bellafill_e-IFU_US.pdf
- Suneva Medical Inc. Bellafill Skin Test Instructions for Use [Internet]. [cited 2020 Jun 20];Available from: https://bellafill.com/wp-content/uploads/2018/12/7252Rev00_Bellafill-Skin-Test_e-IFU_US.pdf